Team Develops Method for Glycosylating Proteins
By Jason Socrates
Bardi
Alfred Hitchcock, who is famous for meticulously planning
every shot of some of his movies before he exposed even a
single frame of film, once remarked that actors should be
treated as cattle—presumably so that they can be herded
through their scenes the way he wanted.
Like making movies, producing glycoproteins—proteins
that have specific carbohydrates (sugar groups) covalently
attached to them—is a process that is sometimes easier
to envision than enact. Molecules, like actors, can be difficult
to work with.
Nature excels at attaching specific sugars to specific amino
acid residues within proteins through posttranslational modification,
and, by some estimates, more than half of all the proteins
in the human body have carbohydrate molecules attached. Posttranslational
modification is widely used by nature to modify the folding,
stability, activity, and interactions of proteins, playing
a central role in cell signaling, development, immunology,
and many other important biological processes.
Scientists would likewise like to have a way to create these
same molecules in the laboratory in order to have an easy
way to examine the effect of glycosylation on the structure
or function of proteins and to develop glycosylated therapeutics,
such as certain antibodies, cytokines, erythropoietin, and
other medicines. These goals have been hindered, however,
by the lack of a convenient and reliable laboratory method
for producing selectively glycosylated proteins.
Now a team of scientists from The Scripps Research Institute
(TSRI) has developed a powerful method that takes a huge step
in the direction of developing such a method. The team was
led by Professor Chi-Huey Wong, who holds the Ernest W. Hahn
Chair in Chemistry, and Peter G. Schultz, who holds the Scripps
Family Chair in Chemistry. Wong and Schultz are also both
members of The Skaggs Institute for Chemical Biology at TSRI.
Using a technology developed in Schultz's laboratory to
site-specifically introduce unnatural amino acids into proteins,
the team has demonstrated the ability to create glycoprotein
"mimetics"—proteins that contain unnatural glycosylated
residues that mimic naturally glycosylated proteins.
In the study, Schultz, Wong, and their colleagues engineered
a codon within a gene that codes for a small domain of the
staphylococcal protein A. In response to this engineered codonan
unnatural amino acid—called p-acetyl-L-phenylalanine
is inserted into the protein. This unnatural amino acid resembles
the natural amino acid phenylalanine but with a "keto" group
attached. The keto group is like a chemical handle to which
the sugars can be attached.
Schultz and Wong found that when they incubated the keto-containing
protein with a sugar solution containing the sugar aminooxy
saccharide, the sugar was covalently attached to the protein
at the end of the day. They further found that they could
sequentially attach a second sugar to the first, and a third
sugar to the second through enzymatic manipulations.
This technology is general enough to synthesize glycoprotein
mimetics of any protein that can be expressed in E. coli,
say the authors.
To read the article, "A Method for the Generation of Glycoprotein
Mimetics" by Haitian Liu, Lei Wang, Ansgar Brock, Chi-Huey
Wong, and Peter G. Schultz, please see J.
Am. Chem. Soc., 125 (7), 1702 -1703, 2003.

|
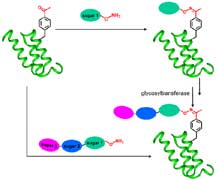
Two routes to sweetening proteins. Schultz,
Wong, and colleagues glycosylated the unnatural amino acid-containing
protein with three sugars. They were able to attach the sugars
both one at a time (clockwise steps) and all at once. Image
by Haitian Liu. Click
to enlarge.
|

