

Tweet
Team Discovers Treatable Mechanism Responsible for Often Deadly Response to Flu
By Anna Sobkowski and Mika Ono
Researchers at The Scripps Research Institute have found a novel mechanism by which certain viruses such as influenza trigger a type of immune reaction that can severely sicken or kill those infected.
This severe immune reaction—called a "cytokine storm"—floods the tiny air sacs of the lungs with fluid and infection-fighting cells, blocking off airways and damaging body tissues and organs. Cytokine storms are believed to have played a major role in the staggering mortality of the 1918-1919 worldwide influenza pandemic, as well as in the more recent swine flu and bird flu outbreaks.
In a new study published in the September 16, 2011, issue of the journal Cell, a team of Scripps Research scientists have pinpointed the cells that orchestrate cytokine storms, opening up entirely new possibilities for treatment of the condition.
"In the new research, we show directly for the first time that the damaging effects of cytokine storm are distinct from the impact of virus replication and pathological changes in infected cells," said Scripps Research Professor Hugh Rosen, MD, PhD, who led the study with Scripps Research Professor Michael B.A. Oldstone, MD. "The findings provide a new paradigm for understanding influenza and could point the way to new therapies."
"This study has greatly increased our understanding of the biological basis of cytokine storm, opening the door to development of new treatments for this potentially fatal immune reaction," said James M. Anderson, MD, PhD, director of the National Institutes of Health (NIH) Division of Program Coordination, Planning, and Strategic Initiatives, which provided funds for the work. "This research is an excellent example of scientific discovery facilitated by the NIH Common Fund's Molecular Libraries and Imaging program and of the potential that this discovery provides for targeted new therapies."
New Approach to an Old Foe
Influenza and many other viruses destroy cells, especially the cells that line the alveoli (tiny air sacs) that exchange gases in the lung. In response, the body generates cytokines (small cell-signaling protein molecules) and brings in a variety of immune cells in an attempt to limit infection. Normally, the production of cytokines is kept in check by the body, but in some cases cytokine production goes into overdrive and results in a dangerous cytokine storm.
Using advanced chemical and genetic approaches that allow tracking and modulation of receptor function in real time, the Scripps Research team set out to determine the role of a receptor S1P1 (molecule on the surface of a cell that binds to molecules, triggering a certain biological effect) for a specific molecule known as Sphingosine-1-phosphate (S1P). S1P1 has been a topic of investigation in Rosen's lab, in part due to its connection to autoimmune disease.
Unexpectedly, the team found that manipulating the S1P1 receptors in the endothelial cells—the thin layer of cells lining the interior surface of blood vessels—in the lung affected cytokine release. Previously, scientists had assumed that cytokine release occurred through virus-infected cells or other cells lining the lungs.
Next, the scientists wanted to see if they could alter the course of cytokine storm in mice infected with the human pandemic influenza strain that resulted in a severe flu season in 2009 (H1N1 2009). Using a molecule that bound to the S1P1 receptor, the team was able to "downregulate" the immune reaction, allowing enough immune response to fight the virus, while at the same time diminishing or even eliminating the cytokine storm and improving survival rate from infection.
"It had been thought for a long time that all injury from influenza was due to the virus itself, consequently, and rationally, the focus was on developing antiviral drugs," said Oldstone. "The surprise in our findings was that by modulating the S1P1 receptors, we could protect the infected host, a target not subject to the rapid mutational escape of the virus, and therefore less subject to resistance."
Looking to the Future
A number of companies including Novartis, Actelion, and Receptos have S1P1 modulators in clinical trials. The Receptos compounds were discovered by the Rosen and Roberts laboratories in the Scripps Research Institute Molecular Screening Center, supported by the NIH Common Fund.
"Now that we know where cytokines come from and have isolated the specific receptor-based mechanism, it is likely that a single oral dose of a compound can be developed that will provide protection from cytokine storm early in infection," said Rosen, who added that previous studies from his lab suggested that such a drug could be used in conjunction with Tamiflu, a common antiviral medication used to treat influenza.
Oldstone noted that future research on the S1P1 receptor could help identify which individuals are most susceptible to cytokine storms and would be most likely to benefit from a drug targeting this mechanism. The joint first authors of the study, "Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection," were Scripps Research postdoctoral fellows John Teijaro and Kevin Walsh. Additional authors included Stuart Cahalan, Daniel M. Fremgen, Edward Roberts, Fiona L. Scott, Esther Martinborough, and Robert J. Peach. For more information, see: http://www.cell.com/abstract/S0092-8674%2811%2900941-X
The study was supported by grants from the United States Public Health Service and the National Institute of Allergy and Infectious Diseases, part of the NIH, and the NIH Common Fund. Additional information about the NIH Common Fund can be found at http://commonfund.nih.gov/.
Send comments to: mikaono[at]scripps.edu

"The findings provide a new paradigm for understanding influenza and could point the way to new therapies," says Professor Hugh Rosen. (Photo by BioMedical Graphics.)

"The surprise in our findings was that by modulating the S1P1 receptors, we could protect the infected host, a target not subject to the rapid mutational escape of the virus, and therefore less subject to resistance," says Professor Michael Oldstone. (Photo by Kevin Fung.)

A video of Professors Oldstone and Rosen discussing their research.
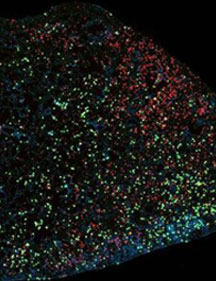
This image shows immune system cells produced by a "cytokine storm" (green and red dots) in lung tissue infected with influenza virus.
