

Tweet
Medically Important Protein Family Yields New Structure
By Laura Bonetta
Scientists from The Scripps Research Institute have determined a new structure from a medically important superfamily of proteins. The structure should help instruct the design of a new kind of therapeutics for conditions ranging from Parkinson's disease to inflammation.
The study, published on March 10, 2011, in Science Express, an advance, online publication of selected research from the journal Science, provides important insights into how this large family of proteins, called G protein-coupled receptors (GPCRs), can recognize and respond to a wide array of signals, including odors, hormones, neurotransmitters, and light.
Many drugs, including allergy and heart medication and drugs for Parkinson's and Huntington's disease, target GPCRs, a family of proteins that comprises some 700 to 1,000 members.
A Surprisingly Stable Active Form
GPCRs sit in the cell membrane and sense various molecules outside cells. When certain molecules bind to them, the receptor's structure shifts so that it transmits its signal within the cell. These receptor-activating molecules are referred to as agonists. But GPCRs can also bind "antagonists," compounds that block the receptors' activity by preventing agonists from binding.
Up until now, researchers had primarily been able to obtain the structures of GPCRs bound to antagonists—in other words, in their inactive but more stable forms. Some scientists thought a receptor bound to an agonist would be too dynamic without stabilizing mutations or G-proteins bound for the receptor to be amenable to forming crystals, a critical step required for determining protein structures using the technique of X-ray crystallography.
In the new study, Fei Xu, a graduate student in the Stevens lab and the first author of the paper, proved these assumptions inaccurate.
The Stevens lab obtained the structure of the human A2A adenosine receptor, a member of the GPCR family sometimes referred to as the "caffeine receptor," bound to a full agonist. The team discovered that when the receptor bound to this particular agonist, it took on a new shape, as expected, but it then remained in that new conformation, rather than continuing to move.
"We were surprised to discover a super stabilizing agonist," said Stevens. "While dynamics is certainly a critical component of receptor signaling, it is not as extreme or the complete story as previously thought. The agonist we solved with the A2A structure highlights the fact that certain agonists can stabilize the receptor in a single conformation without the presence of an intracellular binding partner such as a G-protein. This is also teaching us that what we learn from one receptor or one agonist/antagonist interaction should not necessarily be a rule for all GPCRs at this early stage of GPCR structure discovery. We need to study multiple systems in-depth before we will really understand this receptor family."
The finding has important implications for drug design. In some diseases, such as Parkinson's disease, potential treatments involve blocking the functions of certain GPCRs using antagonists. But for treating other diseases such as COPD, researchers are trying to develop agonists that activate GPCRs. This new finding could facilitate the design of such agonist-based drugs.
Ward Smith, director of the National Institutes of Health (NIH) Protein Structure Initiative (PSI), which funded the study, said, "Determining the structure of the active form of the A2A adenosine receptor represents just the kind of significant accomplishment that the Protein Structure Initiative was intended to foster. Now that we know what the active form looks like, we have a much better idea of how this important class of cellular gatekeepers functions and how we might manipulate their activity in treating disease."
Decades-Long Vision Yields Results
Stevens began working on the structures of GPCRs more than two decades ago. His group in collaboration with researchers at Stanford University solved the first human GPCR structure, the β2 adrenergic receptor, in 2007—a project that took 17 years to complete. Since then the Scripps Research team has been successful in obtaining several other GPCR structures in collaboration with other laboratories around the world.
"The reason we have now solved several human GPCR structures is the strong and robust scientific platform we built at Scripps with NIH support," says Stevens, who is director of the NIH Common Fund Joint Center for Innovative Membrane Protein Technologies, focused on developing and disseminating technologies, and the National Institute of General Medical Sciences PSI:Biology GPCR Network, focused on increasing the knowledge of GPCR biology. "When the NIH funded this research they took a very big chance on high risk/high reward science and it is now paying off in multiple ways from new technologies to new biological insight."
Like all proteins, GPCRs consist of long chains of amino acids that assemble themselves in three-dimensional shapes. GPCRs consist of seven helices that span the membrane of a cell. Loops connecting the helices sit both outside the cell membrane and inside the cell.
In the new study, Stevens and colleagues found that when the agonist bound the A2A receptor, helices 5, 6 and 7 underwent a dramatic shift in their positions. In contrast, helices 1 to 4 tended to stay relatively still. "GPCRs appear to be composed of two domains," he explained. "The first four helices appear more rigid than the last three."
In addition, the portions of the receptor sitting outside the cell membrane shifted their positions to accommodate the agonist binding, propagating changes on the inside of the cell.
The flexibility and diversity for the outside portions of the receptor may hold the key for understanding GPCRs' ability to recognize and respond to molecules of many different sizes and shapes. This is reminiscent of how the immune system uses the antibody architecture to recognize so many different ligands.
"You need receptor diversity on the outside to recognize all the different ligands, but inside the cell, you need less diversity since the receptor signals via a smaller number of binding partners," said Stevens.
In addition to Stevens and Xu, authors of the study, "Structure of an Agonist-bound Human A2A Adenosine Receptor," include Huixian Wu, Gye Won Han and Vadim Cherezov of Scripps Research, Vsevolod Katritch of the University of California, San Diego, and Kenneth A. Jacobson and Zhan-Guo Gao of the NIH in Bethesda, MD.
For more information, see http://www.sciencemag.org/content/
early/2011/03/09/science.1202793 .
Send comments to: mikaono[at]scripps.edu
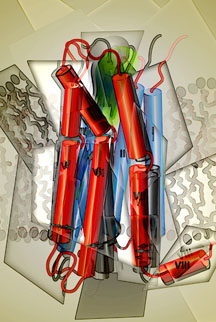
The new study reveals the structure of the human A2A adenosine receptor, a member of the GPCR family sometimes referred to as the "caffeine receptor," bound to a full agonist. See also the molecular movies posted at: http://stevens.scripps.edu/
A2a_Movie.html and http://gpcr.scripps.edu/
A2a_Movie.html .
