

Team Restores Some Function to Cells from Cystic Fibrosis Patients
By Mika Ono
In an encouraging new development, a team led by Scripps Research Institute scientists has restored partial function to lung cells collected from patients with cystic fibrosis. While there is still much work to be done before the therapy can be tested in humans, the discovery opens the door to a new class of therapies for this and a host of other chronic diseases.
The results were published on December 6, 2009 in an advance, online edition of the high-impact journal Nature Chemical Biology.
"We are very excited by these results," said team leader Professor William Balch, a professor in the Departments of Cell Biology and Chemical Physiology and member of the Institute for Childhood and Neglected Diseases, who also receives support from the Skaggs Institute for Chemical Biology, all at Scripps Research. "Because we came at the problem of restoring cell function from a new perspective—using biology to correct biology—these findings have the potential to be game-changing."
The new study, performed in collaboration with a large number of cystic fibrosis investigators across the United States and Canada, showed that a compound called suberoylanilide hydroxamic acid (SAHA), which is already approved by the U.S. Food and Drug Administration as a treatment for lymphoma, can restore about 28 percent of normal function to lung surface cells with the most common, yet severe, cystic fibrosis mutation that results in complete loss-of-function in homozygous patients (those receiving a copy of the mutated gene from both parents).
"The results are very promising," said Balch. "We know that cystic fibrosis individuals with 15 to 30 percent of normal cellular function, as can occur with certain mutations, have milder cases of the disease and a more normal lifestyle than patients carrying a severe mutation. The added degree of function conveyed by SAHA or a compound like SAHA could make a tremendous difference to patients with acute disease."
A Life-Shortening Condition
Cystic fibrosis is an inherited disease that affects about 30,000 children and adults in the United States, and 70,000 worldwide, according to the Cystic Fibrosis Foundation.
In cystic fibrosis, patients produce thick, sticky mucus, which can clog the lungs and result in damaging inflammation and life-threatening infections. This thick mucus can also obstruct the pancreas and interfere with the proper digestion and absorption of food. Other symptoms include diabetes and infertility.
People with cystic fibrosis have mutations in the cystic fibrosis gene, which leads the body to produce a defective protein—called the cystic fibrosis transmembrane conductance regulator (CFTR) protein—which is normally found at the cell surface and is necessary for the proper movement of sodium and chloride (salt) in and out of cells. This process is necessary for the proper hydration of the lung, intestine, and pancreas.
Although more than 1,400 different mutations can lead to defects in CFTR, the most common mutation is a deletion of a phenylalanine residue at position 508 of the protein (ΔF508 CFTR). This mutation, which causes a severe form of the disease, is responsible for more than 90 percent of cystic fibrosis cases worldwide.
While 50 years ago few children with cystic fibrosis lived to attend elementary school, today it is not unusual for people with cystic fibrosis to live into their 30s and 40s. Most treatments available today focus on symptom management—specifically clearing the airways through lung compression vests, antibiotics, inhaled medications, and anti-inflammatory drugs, as well as promoting proper nutrition through a healthy diet, substantive digestive enzyme supplements, and other dietary aids.
Unfortunately, still lacking are approved medications that address problems with the ΔF508 cystic fibrosis protein. (Balch notes that a potentiator drug (Vertex 770) affecting a very rare mutant population of cystic fibrosis patients (G551D CFTR) which is at the cell surface, but unable to mobilize chloride, did achieve significant clinical benefit in a recent clinical trial). A broadly effective therapeutic correcting ΔF508 CFTR protein delivery to the cell surface and restoring function would be a great boon for most cystic fibrosis patients and their families.
Such a development could alleviate the chronic need for the many drugs and therapies that attempt to mitigate the onslaught of symptoms and to enable patients to sustain a normal lifestyle.
Turning a Classical Approach on Its Head
In the new study, Balch took an original approach to correcting cystic fibrosis defects. This new approach grew out of a unique understanding of protein folding and misfolding that Balch had been working out for some time with Scripps Research colleague Jeffery Kelly, chair of the Department of Molecular and Experimental Medicine, Lita Annenberg Hazen Professor of Chemistry, and member of the Skaggs Institute for Chemical Biology. This perspective may also have implications for conditions as diverse as type II diabetes, arthritis, osteoporosis, and amyloid disease (including Alzheimer's).
While in many genetic diseases specific mutations within a particular gene cause the protein product of the gene to misfold, Balch and colleagues note that is not the end of the story. Critically, this defective protein must interact with the general biological machinery of the cell, which controls the protein folding and stability environment. This can contribute significantly to the protein's loss of function and a breakdown in tissue/organism function. This biological machinery controlling the folding and function environment of the cell is referred to as the proteostasis network and is central for life.
"Our network biology approach challenges the current thinking and practices of the pharmaceutical industry that focuses on drugging single targets," Balch said. "This traditional view limits our ability to tackle pharmacologically many complex loss-of-function sporadic and inherited diseases which are really systems disorders. These diseases have multiple steps in the biological network that must be adjusted to regain a more normal function of the compromised protein and tissue."
In the case of cystic fibrosis, Balch suspected that the endoplasmic reticulum—a compartment in the cell responsible for the synthesis of CFTR which normally works to protect the body by degrading potentially dangerous abnormal proteins—could be viewed as doing its job too efficiently, eliminating mutant CFTR proteins that could still provide some function to the cell and tissue if given the opportunity.
In the new Nature Chemical Biology study, Balch and colleagues drew on their previous theoretical and experimental work to turn the classical model of drug development on its head. Rather than attempting to directly target or replace the mutant CFTR proteins present in cystic fibrosis patients—an approach that had so far failed to yield dramatic new treatments for the ΔF508 disease—Balch and colleagues sought instead to adjust the cell folding or maintenance proteostasis machinery of the cell to make a new cellular environment that would "work" with the mutant CFTR proteins.
In so doing, the scientists hoped that the mutated CFTR proteins, while not perfect, could now function more effectively in the cell, reducing the more severe effects of cystic fibrosis symptoms in the common ΔF508 variant.
Positive Results on Multiple Levels
To tweak the cellular machinery in this fashion, Balch and the team enlisted compounds that were known to inhibit a family of enzymes known as histone deacetylases (HDACs). Studies have shown that HDACs affect the packaging of the DNA in chromosomes and regulate gene expression. Based on earlier studies of the folding environment required for CFTR function published in the journal Cell, the scientists reasoned that altering HDAC function might also rebalance proteostasis networks in the cell to favor functional restoration.
For expertise in these compounds, Balch teamed up with Scripps Research colleague Professor Joel Gottesfeld and his group.
"Joel's lab and my lab worked very closely together on this," said Balch. "That's what's great about Scripps Research—its collaborative nature. People just walk in next door and two hours later you are doing an experiment together!"
Working with Ray Frizzell and Joe Pilewski, cystic fibrosis investigators at the University of Pittsburg School of Medicine, the Balch laboratory treated human lung epithelial cells isolated from patients with the devastating ΔF508 mutation with known HDAC inhibitors. Intriguingly, the FDA-approved HDAC inhibitor SAHA was shown to be most effective in restoring surface channel activity—one of the main markers of cystic fibrosis that is responsible for rehydration of the cells' surface. Control cells demonstrated negligible levels of channel activity, while SAHA-treated cells were restored to 28 percent of the normal level found in healthy individuals.
"It's a pretty solid rescue with some intriguing properties," commented Balch.
Using a bioinformatics approach led by the Gerard Manning laboratory at the Salk Institute of Biological Sciences, the team showed that the compound increased the functioning of mutated CFTR proteins at multiple levels in the proteostasis network. Not only were mutant CFTR proteins more protected from destruction in the endoplasmic reticulum, they were also more efficiently transported to the lung cell surface where they were found at comparable levels to that of wild-type (normal) CFTR. In addition, once at the surface of HDAC inhibitor-treated cells, mutant CFTR proteins were better able to resist destruction by additional degradation pathways than ΔF508 CFTR proteins in untreated cells.
"By rebalancing the proteostasis program to provide a more supportive cellular environment," said Balch, "the cells appear to treat the mutation more like a polymorphism [genetic differences that are responsible for individual diversity] rather than something dangerous needing to be completely eliminated."
Balch also likened this process to evolutionary adaptations to changes in protein structure, which support mutations providing a selective functional advantage.
The study showed that one specific HDAC—HDAC7, one of 18 known human HDACs—appeared to be largely responsible for the effects on the treated cells. Little is known about the function of HDAC7 in human physiology and efforts are currently under way by the laboratories of Balch, Gottesfeld, Manning, and Scripps Research Professor John Yates to understand its mechanism of action.
The Way Forward
Mindful that dosing would be a key issue in any attempts at drug development, the researchers also began to address dosing issues in the current study.
"We know that a compound won't make it into the clinic if patients have to take the equivalent of a cereal bowl of it several times a day," said Balch. "That's especially true because, given the nature of cystic fibrosis, this would need to be a sustained, life-long treatment to protect the patient from disease."
Remarkably, the team found that low doses of SAHA not only worked in cultures of cystic fibrosis lung cells, but also offered some significant advantages over acute doses. While acute doses of SAHA produced an increase in the surface channel activity the next day, the effects stopped soon after the drug was withdrawn. In contrast, much smaller doses began working efficiently after six to eight days and strong channel activity was observed after the drug was withdrawn, gradually declining over the following week. This feature is reminiscent of its potential mechanism of action, Balch said, perhaps involving chromatin remodeling leading to an altered, protective proteostasis environment in the lung cell that could be sustainable.
While thrilled with the results, Balch cautions that "there is much work to do"—including further drug development, preclinical work, and clinical trials—before any new therapy for cystic fibrosis becomes a reality using this approach. The FDA-approved drug SAHA, while initially approved as an acute dose regimen for cancer therapeutics, remains to be carefully examined for use in a low-dose, chronic treatment regimen that would be required for protecting cystic fibrosis patients from disease over a lifetime.
First authors of the paper, titled "Reduced Histone Deacetylase 7 Activity Restores Function to Misfolded CFTR in Cystic Fibrosis," are Darren Hutt and David Herman of Scripps Research. In addition to Balch, Hutt, and Herman, other authors include: Jeanne Matteson, Ben Hoch, Wendy Kellner, Jeffery Kelly, J. R. Yates IIIrd and Joel M. Gottesfeld of Scripps Research; Ana Rodrigues and Gerard Manning of the Salk Institute for Biological Studies; Sabrina Noel, Joe Pilewski and Ray Frizzell of University of Pittsburgh School of Medicine; Andre Schmidt and Philip Thomas of the University of Texas Southwestern Medical Center; Yoshihiro Matsumura and William Skach of Oregon Health and Sciences University; Martina Gentzsch and John R. Riordan of University of North Carolina, Chapel Hill; Eric J. Sorscher of University of Alabama at Birmingham; and Tsukasa Okiyonad and Gergely L. Lukacs of McGill University. Sorscher and Balch are co-chairs of the Cystic Fibrosis Consortium (CFC) a team of investigators that also includes Skach, Thomas, and Lukacs that are supported by the Cystic Fibrosis Foundation Therapeutics (CFFT). The CFC was implemented to encourage cystic fibrosis investigators to collaborate and share results to accelerate the pace for a cure for the disease. Finanical disclosure: Balch is a Consultant and equity holder in Proteostasis Therapeutics Incorporated (PTI), 200 Technology Square, Boston MA, whose goal is to develop drugs that correct human misfolding disease. Kelly is a founder and equity holder in PTI.
This research was supported by National Institutes of Health (NIH) grants HL79442 (WEB/JRY), GM42336 (WEB), and the Cystic Fibrosis Foundation Therapuetics (CFFT) (WEB/JRY); NS055781 to JMG; AG03197 to GM; AG84567 to JWK; DK68196, DK72506, and the CFFT to RAF and JP; UR98647 and the CFFT to ES; DK075302, the Canadian Institutes of Health Research and the CFFT to GLL; DK51818 and the CFFT to WRS; DK23567 to JRR. D. Hutt was supported by fellowships from the Canadian Cystic Fibrosis Foundation and the Canadian Institutes of Health Research; D. Herman was supported by a fellowship from the Friedreich's Ataxia Research Alliance.
Send comments to: mikaono[at]scripps.edu
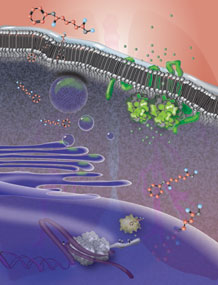
The research team's dramatic new approach to restoring function in cystic fibrosis adjusts the proteostasis program—the folding and function environment—of the cell. Click for details. (Image by Mary O'Reilly.)

"Because we came at the problem of restoring cell function from a new
perspective using biology to correct biology these findings have the
potential to be game-changing," says Professor William Balch (right), shown
here with co-authors Professor Joel Gottesfeld (left), Research Associate
David Herman (second left), and Research Associate Darren Hutt.
