

Scientists Find Key Culprits in Lupus
By Laura Bonetta
The more than 1.5 million Americans with systemic lupus erythematosus (or lupus) suffer from a variety of symptoms that flare and subside, often including painful or swollen joints, extreme fatigue, skin rashes, fever, and kidney problems. Researchers at The Scripps Research Institute have now identified the main trigger for the development of this disease.
Lupus is one of several autoimmune diseases in which the immune system turns against parts of the body, destroying the very cells and tissues it is meant to protect. In a study published in the Early Edition of the Proceedings of the National Academy of Sciences (PNAS) July 2, 2009, Scripps Research Professor of Immunology and Microbial Science Dwight Kono and colleagues demonstrate that three proteins, called Toll-like receptors (TLRs), are necessary for this autodestruction to occur. TLRs may thus provide effective targets for the development of new treatments for lupus, as well as other autoimmune diseases.
The Double-Edged Sword of Immunity
In response to infection, a healthy immune system produces antibodies—proteins that fight and destroy invading pathogens such as viruses, bacteria, and other foreign substances. But in lupus something goes awry with the chain of events leading to antibody production. As a result, the immune system produces "autoantibodies" against some of the body's own molecules, cells and tissues.
TLRs are proteins found in immune cells that normally help stimulate the initial response of the immune system to foreign pathogens. Humans have 10 different types of TLRs. Some of them sit on the surface of immune cells and seek out molecules that appear on the coating of bacteria and viruses. Other TLRs—TLR 3, TLR7, (TLR 8 in humans, but not mice), and TLR 9—reside inside immune cells, in a compartment known as the endolysosome, where bits of foreign substances usually end up.
When bacteria or viruses enter the body, some are engulfed by immune cells and degraded in the endolysosome. Inside this compartment, resident TLRs come across the bacterial and viral debris. These TLRs specifically detect the genetic material of pathogens—viral DNA, viral RNA, and bacterial DNA—and stimulate immune cells to produce antibodies against these molecules.
But the production of antibodies against foreign DNA and RNA seems to be particularly prone to error. The most common types of autoantibodies found in lupus patients are ones to the body's own genetic material—the DNA and RNA that resides inside the cell's command center, or nucleus. As a result, doctors often test for the presence of "antinuclear" antibodies to diagnose lupus.
"That's the Achilles heel," says Kono. "These endolysosomal TLRs are needed for viral and bacterial immunity, but they open the possibility of self reactivity."
Toll-Like Receptors and Lupus
Scientists don't quite know how antinuclear antibodies develop, but have suspected for some time that TLRs might be involved. By engineering mice that lack either TLR 7 or TLR9, scientists have gathered evidence that these TLRs may play a role in the disease.
"Earlier studies had strongly suggested that endolysosomal TLRs were important, but if you eliminate one or the other you do not get a huge effect," says Kono. "So we asked, 'What happens if you get rid of all the endolysosomal nucleic acid-sensing TLRs at once?'"
To answer this question, Kono and colleagues took advantage of strains of laboratory mice prone to lupus. These mice spontaneously develop many of the same signs and symptoms as humans with the disease. The next step was to eliminate TLR 3, TLR 7, and TLR 9 in these lupus-prone mice.
But how do you get rid of three proteins at once? Kono and colleagues knew that these TLRs need to be transported to the endolysosome to function. They also knew that one particular protein, called UNC-93B, produced by a gene called Unc93b1, serves as an essential "taxi" service. The UNC-93B protein attaches itself to TLR 3, TLR 7, and TLR 9 and facilitates their transport from the compartment in the cell where they are made to the endolysosome.
Using geneticists' tools of the trade, Kono and colleagues, engineered lupus-prone mice with an inactive Unc93b1 gene. Compared to lupus-prone mice with a functioning Unc93b1 gene, the mice with the Unc93b1 mutation produced fewer antinuclear antibodies and had fewer and less severe symptoms of lupus.
As a further test, Kono and colleagues treated the mutant mice with a substance that stimulates TLR 4—as TLR 4 stimulation is known to promote the production of autoantibodies. But even with TLR 4 stimulation, the mice lacking functioning TLR 3, TLR 7, and TLR 9 did not develop lupus.
"It seems like these three TLRs are absolutely required for optimal autoantibody production," says Kono. "This is an important finding that builds on results obtained by other groups."
The results "suggest that the three endosomal TLRs, or UNC-93B itself, might be good targets for therapy," says Kono, adding that more tests will be needed before these findings are translated into treatments for patients. "We are definitely getting closer to understanding the etiology of this autoimmune disease."
In addition to Kono, other co-authors of the article "Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus," include M. Katarina Haraldsson, Brian R. Lawson, K. Michael Pollard, Yi Ting Koh, Xin Du, Carrie N. Arnold, Roberto Baccala, Bruce Beutler, and Argyrios N. Theofilopoulos of The Scripps Research Institute, and Gregg J. Silverman of the University of California, San Diego. For more information, see http://www.pnas.org/content/early/2009/07/01/0905441106.abstract.
This research was supported by the National Institutes of Health.
Send comments to: mikaono[at]scripps.edu

"It seems like these three Toll-like receptors are absolutely required for optimal autoantibody production," says Professor Dwight Kono (right), pictured here with co-author Argyrios Theofilopoulos, chair of Immunology and Microbial Science. Photo by Kevin Fung.
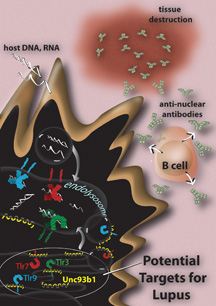
Toll-like receptors may provide effective targets for the development of new treatments for lupus and other autoimmune diseases. Illustration by Mary O'Reilly. Click for details and to enlarge
