

Research Unveils New Stabilizing and Signaling Properties of Cholesterol in Key Human Receptors
By Eric Sauter
Scientists at The Scripps Research Institute have structurally shown for the first time that cholesterol acts as a stabilizing factor for an important family of cell receptors.
Based on these findings, the study predicted that more than one quarter of G-protein-coupled receptors have specific cholesterol binding sites and that these sites might be targets for the development of new therapeutics.
The study was published in the June 11, 2008 edition (Volume 16, Issue 6) of the journal Structure.
"Our study shows that cholesterol is a key structural element of a very important family of cell signaling receptors," said Ray Stevens, a Scripps Research scientist and professor in the Department of Molecular Biology. "We believe that these newly discovered sites will be major targets for the future discovery and development of therapeutics. That's what we're currently investigating."
Until this study, it was believed that cholesterol's main membrane protein influence was on membrane fluidity and curvature.
"Not only does cholesterol provide stability but its presence has been shown to increase the affinity of various agonists in other receptors–serotonin, for example," Stevens said. "The question right now is to determine just how much of an effect cholesterol has on the receptor stability and function. We need to further validate the relationship between the newly discovered cholesterol sites and the ligand binding sites on these receptors."
G protein-coupled receptors, with more than one thousand members, is the largest and most diverse protein family in the human genome. They transduce or convert extracellular stimuli into intracellular signals through a number of signaling pathways, including neurotransmitters, light, hormones, lipids, and proteins. Because of their diverse signaling pathways, approximately one third, and perhaps as many as half, of currently marketed drugs are designed to target these receptors.
The Use of Crystal Structures
In the study, Stevens and his colleagues determined the crystal structure of human β 2-adrenergic receptor, which led to a high-resolution view of a human G protein-coupled receptor bound to a pair of cholesterol molecules and timolol, a first-generation beta-blocker that has been used extensively in the treatment of glaucoma, high blood pressure, and heart disease.
"What no one appreciated when we solved the first GPCR crystal structure was the importance of cholesterol," he said. "Most thought that it was probably an artifact of the crystallization process. This time around we were able to develop biochemical and structural data to prove that cholesterol uniquely and specifically binds to the receptor."
What Stevens and his colleagues found was unambiguous structural evidence of specific cholesterol-binding sites.
"In this high-resolution structure," he said, "we discovered that the cholesterol molecules specifically bind away from any crystal packing regions and also demonstrated that cholesterol has an effect on the thermal stability of the receptor in the presence and absence of timolol, and that the incorporation of cholesterol into cell membranes modifies the ligand binding properties of the receptor.
"The effect of cholesterol binding on the properties of any receptor will vary across class and will have to be determined for each one," he continued. "So, while future studies will be needed to determine the actual importance of these positions in cholesterol binding, it is increasingly apparent that sterols play an important direct role in the stability and pharmacology of adrenergic receptors."
The study noted that receptors for oxytocin and serotonin (both neurotransmitters) are also biochemically affected by cholesterol. In both receptors, the addition of cholesterol molecules led to a dramatic increase in agonist affinity; for oxytocin, it also increased stability.
Determination of the initial structure of carazolol bound β 2-adrenergic receptor was listed as one of the top ten breakthroughs of 2007 in the journal Science. Stevens and his colleagues worked with Professor Brian Kobilka and other scientists from Stanford University School of Medicine on the decades-long project.
Other authors of the study, A Specific Cholesterol Binding Site Is Established By The 2.8 Å Structure Of The Human β 2-Adrenergic Receptor, include Michael A. Hanson, Vadim Cherezov, Mark T. Griffith, Christopher B. Roth, Veli-Pekka Jaakola, Ellen Y. T. Chien, Jeffrey Velasquez, and Peter Kuhn of The Scripps Research Institute. For more information, see http://www.structure.org/content/article
/abstract?uid=PIIS0969212608001743
The study was supported by the National Institutes of Health as part of the RoadMap Joint Center for Innovative Membrane Protein Technologies.
Send comments to: mikaono[at]scripps.edu
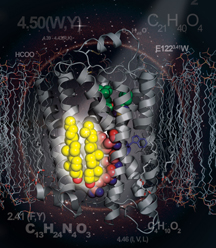 This image shows the structure of cholesterol (yellow) bound to a human β 2-adrenergic receptor (gray) in the membrane. Highlighted in green is timolol, a drug approved by the U.S. Food and Drug Administration for treatment of high blood pressure, heart disease, migraines, and glaucoma.
This image shows the structure of cholesterol (yellow) bound to a human β 2-adrenergic receptor (gray) in the membrane. Highlighted in green is timolol, a drug approved by the U.S. Food and Drug Administration for treatment of high blood pressure, heart disease, migraines, and glaucoma.
