

New Chemical Tool Captures Previously Unseen Molecules
By Mark Schrope
Chemistry, like so many things in life, is rarely as neat as it might appear on paper. Chemists are well aware that real-world reactivity is not as simple and neat as chemical formulas might suggest. For instance, detecting the many intermediate chemical compounds typically created on the way from one side to the other of a formula has always been challenging because these compounds are generally produced in vanishingly small quantities, and last for very short periods.
But in the July 27 issue of Science, Julius Rebek, professor at The Scripps Research Institute and director of its Skaggs Institute for Chemical Biology, and colleagues describe a new chemical tool that effectively pauses the formation of certain intermediate products never before seen, allowing them to be identified and studied.
The tool is a new modification of a synthetic molecule known as a cavitand, which researchers have used for decades to capture a variety of small molecules. While past cavitands were typically bowl shaped, the Scripps Research team has designed a cavitand that has deeper walls, more like a bucket, to help hold captured chemicals. The team was also able to add a feature Rebek calls the chemical equivalent of a fishing pole with a baited hook.
When specific intermediate chemical compounds enter the new cavitand, they "take" the bait, a reactive aldehyde group, and ultimately bond reversibly with the cavitand's bucket, which then envelopes the captured molecule. The cavitand reacts with organic chemicals that contain amines, which includes amino acids and a range of other biologically essential compounds. The process effectively isolates captured molecules from the surrounding medium, preventing them from reacting to form more stable final products for minutes to hours, which is long enough to allow imaging using nuclear magnetic resonance (NMR).
Eventually, the compounds' bonds with the cavitand rupture, allowing the product to move back into the medium and the cavitand to temporarily capture another molecule. Because this bonding and release process does not alter the chemical products in question, it does not alter the normal chemical reaction, but simply slows it.
"This cavitand allows you to see things you can't see any other way," says Rebek, "giving you a picture of what happens to molecules when they're at very close range."
Rebek says such illumination of elusive chemical intermediates improves basic understanding of chemical processes, and he envisions a number of potential applied uses for the work as well.
With further development, one possibility, which the Rebek team is already pursuing in collaboration with researchers at Marquette University in Milwaukee, would be to use the new cavitand to capture and effectively separate chemical isotopes, which could for instance aid biosynthesis studies.
Another option would be to use the molecules to mimic enzymatic reactions, which could help advance drug development studies. Many enzymes work by temporarily binding to their substrates in ways that can't currently be detected. But, says Rebek, "We can set up that sort of situation inside these cavitands," which could help researchers better understand how to harness or alter important enzyme reactions.
Different forms of cavitands are already being used to detect pollutants such as benzene, and Rebek also sees similar applications for the new cavitand.
Future research for the Rebek group will include developing further cavitand modifications that will allow work with an expanded range of chemicals and enable studies of molecules with ever-shorter lifetimes. "The fishing pole is there," says Rebek, "but we can change the nature of the hook—for instance, we could add the equivalent of a spear gun on it, or a revolver." Such modifications, he says, should result in attracting and holding in place different kinds of molecules.
In addition to Rebek, authors of the article, "Stabilization of Labile Carbonyl Addition Intermediates by a Synthetic Receptor," are Tetsuo Iwasawa and Richard Hooley. See Science at http://www.sciencemag.org/cgi/content/abstract/317/5837/493.
The research was supported by the Skaggs Institute and the National Institutes of Health.
Send comments to: mikaono[at]scripps.edu
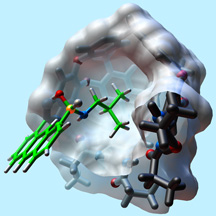
Members of the Rebek lab have found a way to stabilize and isolate reactive intermediates in a synthetic receptor. Click for image details.
