

New Study Reveals Mechanism Vital to Cell Division Regulation
By Eric Sauter
While the small protein GTPase RhoA has long been considered fundamental to cytokinesis, the final division of the cell after mitosis, the actual mechanism of its activation has never been fully understood. Now, a group of Scripps Research Institute scientists led by Professor Gary Bokoch has finally identified a key protein that regulates RhoA activation during cytokinesis and has linked its regulation to mitotic kinases that ultimately control the entire activation process.
The new study, the result of collaboration between scientists at Scripps Research, the University of California, and the University of North Carolina, was published in the May 8, 2007 (Volume 12, Issue 5) edition of the journal Developmental Cell.
In the study, Bokoch and his colleagues clearly establish the role of the guanine nucleotide exchange factor (GEF) H1 as the primary activation mechanism for RhoA, as well as demonstrating the supporting role played by Ect2, another regulatory protein involved in concentrating RhoA along the equatorial line of the dividing cell. During cytokinesis in mammals, the timed regulation of RhoA activity at sites of cell cleavage will eventually lead to the splitting of the cell in two. The new study is the first to link these complementary proteins.
By identifying GEF-H1 as the primary RhoA activator, Bokoch has not only added a significant new insight to the field of cell division but has also uncovered a potentially powerful target for the next generation of cancer therapies, one that could interrupt the process of cell division at a key point. Overexpression of RhoA has been linked to a number of cancers including breast, colon, lung, and certain types of testicular and head and neck cancers. GEF-H1 itself was originally identified in a screen for proliferation-regulatory genes and, soon after, as a transforming oncogene. Increased GEF-H1 expression is strongly linked to the status of the tumor suppressor p53, thereby accelerating cell proliferation. Conversely, while abundant in leukocytes, GEF-H1 is one of only six genes down-regulated in cancer patients treated with the chronic myelogenous leukemia drug, Gleevec.
"During the late phase of cell division, after chromosomes have split into sister sets, the cell forms the cleavage furrow, a process that leads to pinching off the newly separated nuclei," Bokoch said. "There are two important signals involved in the formation of the cleavage furrow: a decrease in the density of the microtubules, which are structural components of the cytoskeleton, in the area of the cleavage furrow, and the activation of RhoA."
It was the change in microtubule density, Bokoch said, that led postdoctoral fellow Jórg Birkenfeld to take a much closer look at the role of GEF-H1. Previous work from the Bokoch laboratory showed that GEF-H1 activity is regulated by microtubule binding, leading the scientists to speculate that GEF-H1 may be the "missing link" that couples microtubule reorganization during mitosis to RhoA activation.
"Our study verifies the involvement of GEF-H1 in the RhoA activation process," Bokoch said. "For example, when you modify or inhibit GEF-H1 function, you get a number of serious mitotic defects." Among the defects observed in the study were asymmetric furrowing, membrane fragmentation, and impaired cytokinesis.
In gaining a clearer picture of the process, the scientists used fluorescence-based probes of Rho GTPase activity in live cells that could be imaged in real time during RhoA activation. In doing so, they were able to finally determine the two very different roles of GEF and Ect2 during cytokinesis.
"Being able to actually see RhoA in live cells was unique and critical to the study," Bokoch said. "When we modified or knocked out the activity of GEF-H1, we found minimal RhoA activation. In contrast, when we depleted Ect2, we still saw RhoA activity but only around the periphery of the cell, not in the cleavage furrow. From that information, we concluded that GEF-H1 is the catalyst for RhoA activation, while Ect2 has a supporting role in localizing activity to the cleavage furrow area. Our work indicates that Ect2 is not likely to be the major activator of RhoA in mammalian cell cytokinesis."
The study also uncovered the role of mitotic kinases—Aurora A/B and Cdk1/Cyclin B—that inhibit GEF-H1 activity through the process of phosphorylation (the addition of a phosphate group to a protein), which can change both the polarity and shape of a protein, thereby switching it on or off. In the case of GEF-H1, the mitotic kinases manage to provide a critical "timer" within the framework of the mitotic process.
"These kinases coordinate GEF-H1 activity so that it is turned on and off at the appropriate time," Bokoch said. "Imagine if, when the cell starts to divide, suddenly a great of amount of active GEF-H1 were released into the dividing cell. That could seriously disrupt the entire process. We believe as soon as GEF-H1 is released from the microtubules, it is phosphorylated by the Aurora kinase, inhibiting its activity. However, as cell division progresses to the cytokinesis stage, the phosphate group is removed and GEF-H1 becomes active again at precisely the right time to help initiate and complete the final cell division process".
Other authors of the study, GEF-H1 Modulates Localized RhoA Activation during Cytokinesis under the Control of Mitotic Kinases, include Jórg Birkenfeld, Perihan Nalbant, and Benjamin Bohl of The Scripps Research Institute; Oliver Pertz of the University of California, San Diego; and Klaus M. Hahn of the University of North Carolina.
The study was supported by the National Institutes of Health, the Deutsche Forschungsgemeinschaft, and the American Heart Association.
Send comments to: mikaono[at]scripps.edu
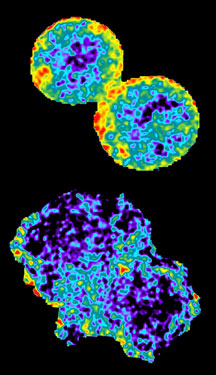
An image from the Bokoch lab shows localized activation of RhoA in living cells during cytokinesis (warmer colors represent higher activity). The upper image shows a control cell; the lower image depicts a cell depleted of GEF-H1 using siRNA.
