

Glucose "Sensor" Plays Dual Role in Glucose Metabolism and Fat Synthesis
By Eric Sauter
In a new study, scientists at The Scripps Research Institute and the Genomics Institute of the Novartis Research Foundation (GNF) have described for the first time a glucose activated sensor that acts as a switch to decrease production of endogenous glucose in the liver, and increase conversion of glucose to fat for storage in adipose tissue. This dual action makes the sensor, Liver X Receptor, a potential target for new therapies aimed at obesity and diabetes. The research may also have implications for heart disease and stroke.
The study was published December 24 in an advanced, online edition of the journal Nature.
In the study, glucose is shown to stimulate the activity of the Liver X Receptors (LXR) a and b, The LXRs act as sensors of dietary components, orchestrating the body's response to nutrients such as oxysterols (short-lived derivatives of cholesterol) and controlling gene expression linked to cholesterol and fat metabolism.
"When you eat, glucose pours into the gut and is recognized by LXR in the liver, which then activates expression of the enzymes that turn excess glucose into triglycerides that are stored as fat," said Assistant Professor Enrique Saez, a Scripps Research scientist who led the study, which was supported by GNF. "The fact that our study demonstrates that LXR does both—it binds to glucose and it induces fatty acid synthesis—is significant and makes LXR a potential target for diabetes and obesity treatments."
In some recent animal studies, Saez pointed out, activation of LXRs using synthetic molecules also induced regression of atherosclerosis, the clogging, narrowing, and hardening of the body's large arteries and blood vessels that can lead to stroke, heart attack, and eye and kidney problems. Elevated levels of pathogenic cholesterols, also known to bind LXR, are a primary risk for development of atherosclerosis.
"The integration of glucose sensing and control of lipogenesis by LXR may explain why low-fat/high-carbohydrate diets induce hypertriglyceridemia [an elevated level of triglycerides in the blood]," Saez said. "LXR can sense surplus glucose, induce fatty acid synthesis, and prompt the liver's export of triglycerides into the bloodstream. Since LXR acts as the body's sensor of a buildup of pathogenic cholesterol, its ability to bind both glucose and oxysterols suggests that LXR may be a link between hyperglycemia and atherosclerosis."
In fact, Saez and his colleagues originally looked at LXR as a drug target for atherosclerosis. But when they fed synthetic LXR ligands to mice to induce activation, they discovered that the mice metabolized glucose more effectively and that activation suppressed new production of glucose in the liver.
That prompted the scientists to look more closely at glucose levels as the LXR activating mechanism in the liver.
To their surprise, what Saez and his colleagues discovered was that glucose bound directly to LXR. This was unexpected because the carbohydrate does not conform to the standard definition of a typical ligand that activates nuclear receptors, transcription factors that coordinate gene expression in response to hormonal and environmental signals. This discovery, Saez said, represents the first signaling pathway where a carbohydrate activates a nuclear receptor, although the precise mode of binding remains unknown.
As part of the study, mice were put on exclusive sucrose or D-glucose diets; all diets were devoid of cholesterol to minimize naturally occurring oxysterols. D-glucose and GW3965 (a synthetic LXR activator) induced similar changes in hepatic gene expression, indicating that LXR functions as a glucose sensor in vivo that responds to increasing liver glucose uptake. The ability of the LXRs to respond to glucose and its derivatives was very specific: no effect was seen in other nuclear receptors tested.
The current study focused primarily on the role of glucose sensing in the liver and gut. New studies will focus on the question of whether glucose levels in other tissue types, such as the pancreas, activate LXR, Saez added.
Other authors of the study, TheNuclear Receptor LXR is a Glucose Sensor, include Nico Mitro of Scripps Research and GNF; and Puiying A. Mak, Leo Vargas, Cristina Godio, Eric Hampton, Valentina Molteni, and Andreas Kreusch of GNF. See the journal Nature at: http://www.nature.com/nature/journal/vaop/ncurrent/abs/nature05449.html.
Send comments to: mikaono[at]scripps.edu
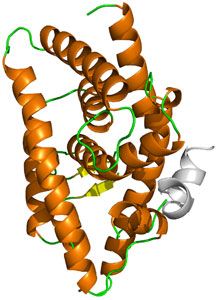
Structure of human LXRα showing the pocket where activators typically bind.

Cell biologist Enrique Saez led the recent Nature study, which suggests potential new targets for obesity and diabetes therapies.
