

Scientists Identify Cells That Promote Repair of Blood Vessels in the Eye
By Anna Sobkowski and Mika Ono
Scientists at The Scripps Research Institute have discovered a method of repairing and normalizing blood vessels in the eye through the use of stem cells derived from bone marrow. These findings may point to a new approach for developing treatments for certain eye diseases.
The research, led by Scripps Research Professor Martin Friedlander, was published online November 16 in The Journal of Clinical Investigation.
In the new study, the team injected immature white blood cells from bone marrow—myeloid progenitors—into eyes with an abnormal vasculature (network of blood vessels) in a mouse model developed to mimic certain human disorders. The researchers found that not only did the progenitor cells migrate to avascular areas of the retina, but once there they differentiated into cells called microglia that actively promoted vascular repair.
"From a purely basic science perspective, this is a novel observation," says Friedlander. "Even more importantly, the study introduces the idea that bone marrow-derived myeloid progenitors could be used to treat ischemic eye disease—an entirely new paradigm."
While there had been increasing interest in microglia among scientists in the field, this is the first time microglia have been shown to contribute to the promotion of vascular repair in any organ, including the eye. These results suggest that it might be possible to use cells derived from a patient's own bone marrow or cord blood to treat such eye diseases as diabetic retinopathy or retinopathy of prematurity (ROP).
Diabetic retinopathy in adults and ROP in pre-term infants are both characterized by abnormal vasculature in the eye. While less common today with the development of better premature newborn infant care, ROP remains a significant cause of vision loss in premature children. Complications from diabetes are the leading cause of vision loss for individuals under the age of 55 in industrialized nations, accounting for vision loss in over 40,000 Americans each year. In total, 2-3 million Americans suffer vision loss from neovascular eye disease such as diabetic retinopathy, ROP and macular degeneration.
In these cases, the development of abnormal vasculature appears to be a response to ischemia—restrictions in blood vessels and insufficient oxygen in the tissue—leading to the formation of extra blood vessels in the back of the eye. But, unlike in the heart or brain, where the extra blood vessels can have a benefit, the new vessels wreak havoc in the eye, leaking fluid and blood and leading to vision loss.
"The diabetic eye, like other tissues in these patients, is starved for oxygen due to the vascular abnormalities associated with diabetic blood vessels," Friedlander says. "New blood vessels form as the body's misguided response to the need to bring more blood and oxygen to the back of the eye."
Current treatments for these eye diseases—such as thermal lasers and anti-angiogenic drugs—are designed to prevent the growth of new vessels or to close, ablate, or remove abnormal vessels. These treatments often fail to completely inhibit abnormal vascular growth, and may cause tissue injury and even exacerbate the underlying ischemia that drives many of these pathological processes.
In this new research, Friedlander and his group have demonstrated that it is possible to approach treatment for these vascular diseases of the eye in an alternative way—by repairing, rather than destroying, blood vessels, addressing the underlying cause of the problem—a lack of oxygen in the tissue.
"The use of progenitor cells would hopefully enable us to rebuild healthy vasculature that could reduce the ischemia driving these conditions, saving mature blood vessels that would otherwise leak fluid and/or proliferate causing vision loss," he says. "By preserving existing vessels and normalizing the new ones, we hope to provide the eye with the oxygen it needs so it won't have to grow new and potentially damaging vessels."
Friedlander and his group at Scripps Research began studying the use of adult bone marrow-derived progenitor cells for the treatment of eye diseases about six years ago. In 2000, they showed, for the first time, in mouse models that these cells could target, and even integrate into, the vasculature in the back of eyes that were developing normally. They went on to further demonstrate that these same progenitor cells, when targeted to degenerating blood vessels, could stabilize and prevent deterioration of vasculature that would otherwise degenerate. The progenitor cells selectively targeted neuronal support cells called astrocytes, which help guide newly forming blood vessels both during normal development as well as in many eye diseases such as diabetic retinopathy or retinopathy of prematurity. By engineering the cells to express anti-angiogenic molecules, the scientists were able to target sites of angiogenesis and prevent blood vessel proliferation. The results of this work were published in Nature Medicine in September 2002.
The group next found that in addition to targeting and stabilizing blood vessels that would degenerate in the eye, the bone marrow cells also had a "neurotrophic rescue effect" in which neurons were also rescued as well as blood vessels. This inherent neurotrophic rescue effect of the targeted cells has potential application in the treatment of the disease retinitis pigmentosa, an inherited degenerative eye disease. The results of this work appeared in The Journal of Clinical Investigation in 2004.
With the most recent findings, the Friedlander team has advanced the progenitor cell work by defining and purifying an active subpopulation, discovering that myeloid progenitor cells from bone marrow can differentiate into microglia and showing that microglia can rescue blood vessels in the eye.
In addition to Friedlander, authors of the paper, titled "Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy," are Matthew R. Ritter, Stacey K. Moreno, Edith Aguilar, and Michael I. Dorrell of Scripps Research, and Eyal Banin of Scripps Research and Hadassah-Hebrew University in Jerusalem.
This work was supported by the National Eye Institute of the National Institutes of Health, The Robert Mealy Program for the Study of Macular Degenerations, the V. Kann Rasmussen Foundation, The MacTel Foundation, the Kovner Family Fund, and the Scripps Fonseca/Mericos Fund.
Send comments to: mikaono[at]scripps.edu
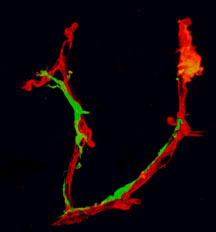
The new study introduces the idea that myeloid progenitor cells from bone marrow could be used to treat some types of eye diseases. Click for details
