

Researchers Unveil Strategy for Creating Actively Programmed Anti-Cancer Molecules
By Eric Sauter and Mika Ono
Scientists at The Scripps Research Institute have developed a unique assembly strategy to produce an anti-cancer targeting antibody, an approach that combines the merits of small molecule drug design with immunotherapy. Among the potential therapeutic advantages is a dramatically increased circulatory half-life of the compound, which could give patients greater exposure to the benefits of any treatment.
The new study, which was published July 5 in an advanced, online edition of the Proceedings of the National Academy of Sciences, achieved a significant enhancement of the treatment of metastatic breast cancer in animal models. The study showed the new hybrid compound remained in circulation for a week. In comparison, the small molecule drug was cleared in a matter of minutes.
"Although the study focused specifically on breast cancer, these new findings could have broad application in the treatment of a number of other cancers, potentially increasing the efficacy of a number of existing or undeveloped small molecule therapies," said Subhash C. Sinha, associate professor in the Scripps Research Department of Molecular Biology and a member of the Skaggs Institute for Chemical Biology, who led the research with Scripps Research President Richard A. Lerner, M.D., Lita Annenberg Hazen Professor of Immunochemistry, Cecil H. and Ida M. Green Chair in Chemistry, and a member of the Skaggs Institute for Chemical Biology.
In the study, the scientists created what is known as a "chemically programmed antibody" by using small cell-targeting molecules and a non-targeting catalytic monoclonal aldolase antibody in a novel self-assembly strategy. Antibodies are proteins produced by immune cells that are designed to recognize foreign pathogens harmful to the body; monoclonal antibodies are produced in the laboratory from a single cloned B-cell, the immune system cell that makes antibodies.
"By bringing together chemistry and biology, our approach provides a way to break the traditional one antibody-one target axiom of immunochemistry," said Lerner. "This new hybrid technology offers great possibilities for the enhanced treatment and diagnosis of a variety of diseases, including cancer."
Preventing Breast Cancer Metastasis
In the study, the researchers used the unique assembly strategy to create a novel compound to combat metastatic breast cancer.
Breast cancer can be a treatable disease, but only if diagnosed early. Since the prognosis grows considerably worse once the cancer has spread to other organs, the prevention of metastasis—the phenomenon in which cancer cells separate from a tumor mass, move through the bloodstream, anchor down in a distant tissue or organ to begin a new cancer—remains a critically important goal. The most recent figures from the Centers for Disease Control and Prevention indicate that more than 200,000 U.S. women are diagnosed with new cases of invasive breast cancer each year and that more than 40,000 of these women will die of the disease—usually the end result of metastasis.
The therapeutic use of monoclonal antibodies has expanded rapidly over the last several years precisely because of their long half-life, combined with their overall lack of toxicity and the fact that they can be easily designed and produced. For cancer treatment, the ability of antibodies to direct immune system responses to specific tumor types is one of the factors that have led to their increased use.
Until recently, it had been widely accepted that while antibodies possess a number of therapeutically advantageous traits, treatment with monoclonals required a different antibody for each specific target. However, the paper's authors have been showing that scientists can use different small molecule targeting agents—called programming agents or adapters—to selectively direct the same antibody to different sites for different uses so that only a single antibody is required for multiple tasks.
Recent research led by Scripps Research Professor Carlos F. Barbas III, for example, used the chemically programmed antibody approach in a melanoma model, dramatically enhancing the effectiveness of a small molecule drug (International Journal of Cancer 119 (5), 1194-1207, March 28, 2006).
In the new study, the researchers used a self-assembly process to link small synthetic molecules and a well-known catalytic monoclonal antibody, mAb38C2, through chemical bonding, a modification that resulted in the reprogramming of the specificity of the antibody to match the binding specificity of the small molecule. In this way, the antibodies were directed to the target avb3 integrin—a cell membrane protein expressed on a variety of cancers and the vasculatures they produce.
"Key to this unique approach is the ability of the small molecule targeting agents—called programming agents or adapters—to selectively react in the antibody binding sites without compromising the targeting property of the adapters," notes Sinha.
The results showed those mice treated with the new compound developed significantly fewer metastases than those treated with other similar compounds or the antibody alone. These results, the study noted, were a "significant enhancement in the therapeutic efficacy" of the integrin antagonist directly attributable to the chemically programmed antibody approach.
Moreover, the study indicated that this approach allows for the effective assembly of chemically programmed antibodies in vivo or in vitro, widening the possibilities for their therapeutic application. The compound could be created in vitro and delivered as a conventional single therapy. Alternately, the small molecule and antibody could be injected separately, with the new complex formed in vivo. The authors note that even though the administration of two separate compounds might complicate regulatory approval, the regimen could have advantages—for example, an imaging agent could be attached to the small molecule, allowing a physician to monitor localization of a drug before arming the agent with the antibody molecule.
In addition to Sinha and Lerner, the authors of the new study, entitled "Breaking the one antibody–one target axiom," include Fang Guo and Sanjib Das of The Scripps Research Institute and its Skaggs Institute for Chemical Biology; Barbara M. Mueller of La Jolla Institute for Molecular Medicine; and Carlos F. Barbas III of The Scripps Research Institute and its Skaggs Institute for Chemical Biology.
The study was supported by The Skaggs Institute for Chemical Biology, a grant from the Department of Defense, and the National Institutes of Health.
Send comments to: mikaono[at]scripps.edu
"Although the study focused specifically on breast cancer, these new findings could have broad application in the treatment of a number of other cancers, potentially increasing the efficacy of a number of existing or undeveloped small molecule therapies."
—Subhash C. Sinha
"By bringing together chemistry and biology, our approach provides a way to break the traditional one antibody-one target axiom of immunochemistry."
—Richard A. Lerner
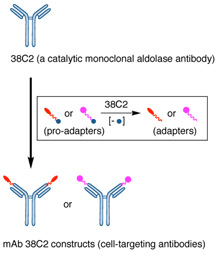
A schematic drawing showing the formation of the cell-targeting antibody constructs from a non-targeting aldolase antibody 38C2 and small molecules equipped with a prolinker.
