

The Bright Side of a Virus
By Eric Sauter
Who would have thought that the humble cowpea mosaic virus would turn out to provide such bounty—despite its natural ability to reduce agronomists to tears over ruined crops?
Scientists at The Scripps Research Institute have developed a positive role for the virus—as a platform for fluorescent dyes to image tissues deep inside living organisms. In a recent study published by the journal Nature Medicine, the researchers used cowpea mosaic virus (CPMV) nanoparticles to light up the vasculature inside mouse and chick embryos and in human tumors. The results were spectacular and on a very small scale.
The researchers found that CPMV—which is only 30 nanometers (a nanometer is one billionth of a meter) in diameter—tagged with fluorescent dyes resulted in exceptionally bright particles. These particles spread throughout the vascular endothelium (the cells lining the interior walls of all blood vessels) for periods of at least 72 hours and produced high-resolution images up to a depth of 500 micrometers (one micrometer equals approximately 1,000 nanometers). Better still, when used in human tumors, the fluorescent CPMV allowed the scientists to clearly identify arterial and venous vessels and to monitor the neovascularization inside what the researchers call the "tumor microenvironment."
By these infinitesimal dimensions, the CPMV is actually close in size to the scale of Fantastic Voyage—the 1966 sci-fi classic where a team of miniaturized surgeons is injected into the human blood stream to save the life of a dying diplomat—but smaller still by about ten-fold, according to Associate Professor Marianne Manchester, one of the study’s authors and a CPMV research pioneer.
The study itself had a serendipitous beginning—over a glass of wine. Manchester and Associate Professor Heidi Stuhlmann happened to be sitting next to one another at a party and the subject of vascular imaging using CPMV came up. Stuhlmann’s laboratory is involved with vascular development—studying how blood vessels develop in embryos and what can go wrong—and she had been looking for new techniques to image live embryo blood vessels. This seemed like the perfect opportunity. Soon, John Lewis, the study’s lead author and a new postdoctoral fellow in the Stuhlmann laboratory at the time, became involved and the idea quickly took hold.
"Sometimes really novel projects are difficult to get underway," Stuhlmann said, "but John came on board and brought a lot of knowledge and enthusiasm for it and got it off the ground."
One of the big problems in obtaining fluorescent vascular images is that most, if not all, of the available agents—liposomes, antibodies, nanospheres, and the wonderfully strange quantum dots (semiconductorcrystals a few nanometers in size)—have been shown to have myriad problems ranging from toxicity to cost. A compatible biological compound like CPMV has a number of distinct advantages, the most important of which is the fact that it is a multivalent compound—the virus has approximately 300 different surface sites to attach just about any kind of molecule scientists want. As result, CPMV is nearly perfect as a kind of tie-dyed projectile for both the clinic and the laboratory.
"There are so many compelling reasons for using CPMV," Stuhlmann said. "First, it has been studied extensively, so we know that it lacks toxicity at high doses and it's very stable and easy to work with. Most importantly, at least for any potential future use, is that it can be grown in large quantities in plants. Add the fact of its multivalency, and it's an ideal candidate for in vivo imaging."
A lot of the early work on CPMV was done at Scripps Research by Professor Jack Johnson, Associate Professor M.G. Finn, and Manchester. It was Johnson who published the first complete CPMV structure in 1986. All three scientists worked together to enhance the virus by attaching various molecules to it, including proteins and peptides.
In this new study, the scientists injected embryos with fluorescent CPMV and found that the brightly lit virus nanoparticles remained internalized by the vascular endothelial cells of the embryos over several days. The same was true of the CPMV injected into adult mice. In both cases, chickens and mice, the images produced using CPMV were "superior to similar-sized fluorescent nanospheres for the resolution of microvasculature during…imaging," the researchers reported.
"Notwithstanding the technical challenges, intravital microscopy affords a view of the complex interplay of cells, signals, and blood flow that can’t be duplicated in a petri dish," Lewis said. "CPMV-based imaging agents are proving to be an extremely useful tool in what is currently a very sparse toolbox for such visualization."
To make the fluorescent CPMV even more bio-acceptable, the researchers coated some of the plant virus with polyethylene glycol (PEG), which further minimizes molecular interactions. This makes CPMV less visible to the immune system, which means it can get to more places in the body without being stopped and asked for ID. That increases the half-life of the nanoparticles in circulation in the vascular system, which improves the imaging. Filmed images from the PEG-coated CPMV are so vivid, in fact, that individual nanoparticles are clearly visible. It was Manchester and Finn who first experimented with coating the virus with PEG to inhibit cellular uptake and immunogenicity.
With either technique, the results of the study point toward the tremendous potential of dye-labeled CPMV to visualize the vascular endothelium, blood volume, and blood flow, as well as malformations in the development of the vasculature in animals, and possibly, humans.
"Our study shows that CPMV nanoparticles are well suited to the visualization of rare molecular targets—and the use of technologies like near-infrared fluorochromes and multiphoton confocal imaging will enhance the quality of imaging at even greater tissue depths," Manchester said. "Remember, viral nanoparticles like CPMV are not limited to fluorescent labeling, so their multivalent properties can be exploited to display a wide variety of molecular tags, including radioactive isotopes, magnetic resonance imaging (MRI) contrast agents, specific enzymatic segments, or any combination of those, and small peptides that act as zip codes directed to specific destinations in the blood vessel network. But that isn't the end. Because virus particles can be manipulated genetically, the door is wide open to future targeted molecular bioimaging studies."
Within the next decade, it may be possible to develop the use of CPMV to image human vasculature, she thinks. It would have to be PEG-coated CPMV to avoid any immune system response, but the same qualities that make the virus ideal for animal studies could potentially work in humans, for example to image an early-stage tumor.
There has already been some outside commercial interest in pursuing the technology further on a preclinical scale.
Authors of the study included John D. Lewis; Giuseppe Destito of Scripps Research and the Dipartimento di Medicina Sperimentale e Clinica, Universitá degli Studi Magna Graecia di Catanzaro, Viale Europa, Campus Universitario di Germaneto, Catanzaro, Italy; Andries Zijlstra; Maria Gonzalez; James P Quigley; Marianne Manchester; and Heidi Stuhlmann.
This study was supported by the National Institutes of Health.
Send comments to: mikaono[at]scripps.edu

Associate Professor Marianne Manchester is one of the authors of a recent study that used cowpea mosaic virus (CPMV) as a platform for fluorescent dyes to image tissues deep inside living organisms.

"There are so many compelling reasons for using CPMV," says Professor Heidi Stuhlmann, also an author of the Nature Medicine paper.
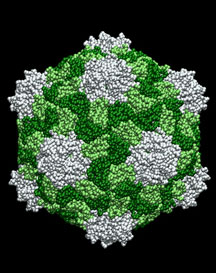 CPMV's advantages include that it has been studied extensively, lacks toxicity at high doses, and is easy to work with. In addition, CPMV can be grown in large quantities in plants—a fact that may be important for potential future uses.
CPMV's advantages include that it has been studied extensively, lacks toxicity at high doses, and is easy to work with. In addition, CPMV can be grown in large quantities in plants—a fact that may be important for potential future uses.
