

Bacillus anthracis: One Bad Bug
By Jeff Worley
Many scientists at The Scripps Research Institute can rightly claim that they've come a long way from their beginnings—humble or not—but Marta Perego's story is hard to top.
"I was born in a nunnery, a 17th-century Italian convent in a little town called Chiavenna on the border with Switzerland," says Perego, an associate professor in the Department of Molecular and Experimental Medicine. "My great-grandfather bought the convent around 1875 and set up a wine merchant business there. My grandfather and father continued the business, and I lived there until I was 19." At that time, Perego left for the University of Pavia, 35 kilometers south of Milan, where she earned a Ph.D. in 1982.
So how does a budding scholar at an 11-century-old European university wind up at Scripps Research?
"At the University of Pavia, I was working in the genetics department, then one of the most advanced in Italy in bacterial science," Perego recalls. "The first DNA sequencing reaction in Italy was being done there." A former University of Pavia postdoc had been working with Professor James Hoch, head of the Division of Cellular Biology in the Department of Molecular and Experimental Medicine at Scripps Research. During a return visit to Pavia, the former postdoc told Perego about the work in the Hoch lab. "Their work was totally exciting to me. When he and Dr. Hoch encouraged me to come to Scripps Research, I jumped at the chance," Perego says.
She began as a postdoc in Hoch's lab in 1984, left for a few years to teach and do research at three Italian universities, then returned to Scripps Research in 1994 to continue her work on the deceptively complex mechanisms of the bacterium Bacillus subtilis.
Working to Tease Out Bacteria's Secrets
Bacteria are the oldest life forms on the planet, and for two billion years the only life on Earth. Structurally simple, these single cells reproduce by fission: they multiply by dividing. Bacteria thrive as the planet's most abundant, most varied, and most useful organisms—and among its most deadly. At the same instant that billions of bacteria are working in our bodies to help digest a meal, others are excavating a cavity in a tooth that our toothbrush has missed. Worse, of course, is the fact that bacteria can kill us.
Perego's recent work has focused on Bacillus anthracis, which can cause anthrax. Humans acquire the disease directly from contact with infected herbivores or indirectly through their waste products. This latter contact accounts for more than 95 percent of cases, although people can also contract intestinal anthrax from eating infected meat, or pulmonary anthrax from inhaling spore-laden dust. This last form of anthrax rode the headlines for weeks in 2001 when anthrax spores were used as a terrorist weapon—distributed through the postal system and causing 22 cases of anthrax, including five deaths.
B. anthracis can exist in two different forms. When conditions for growth are good, with plentiful nutrients and water available, B. anthracis grows and divides. When conditions are unfavorable, each cell forms a resistant dormant spore able to survive extreme environmental conditions.
This spore can be likened to a mummified bacterium. It has a hard protective coating that encases the key parts of the bacterium, and this coating, to continue the analogy, functions like the sarcophagus that protects a mummy within. When conditions become favorable for the bacterium again, it comes to life, transforming from a spore back to a cell. These spores are unimaginably patient: bacterial spores found in the gut of a mastodon in Newark, Ohio, in 1989, were cultured by an Ohio Wesleyan University microbiologist and began to grow—after 11,600 years, according to the carbon dating of the bones.
"What we want to understand in my lab is how this happens," Perego says. "Not just the spore stage, but how and why this bacterium goes from one state to another." She adds that her recent focus on B. anthracis evolved from 20 years of work with Bacillus subtilis, which is similar to B. anthracis except that B. subtilis doesn't cause disease. In her investigations at Scripps Research, Perego has, she says, "the best 'partner-lab' she could imagine." James Hoch, who has worked on B. subtilis for 40 years, is right next door.
Working to Get The Signals Straight
Using what she characterizes as routine molecular biology—cloning a gene, mutating a gene, and expressing a gene to make a protein—Perego is focusing on the signaling mechanisms of B. anthracis to better understand its ability to grow and form spores.
"Any bacterium or any cell, whether it's human or bacterium, is regulated by mechanisms called signal transduction," Perego explains. "A bacterial cell receives signals from the environment or from itself, and it translates those signals into a response."
In other words, something sends the cell a signal—the heat, too much salt—and through a series of chemical reactions transduces this signal to certain proteins that act and activate a response. Signal transduction involves kinases, enzymes that alter other proteins by attaching phosphate to them. Kinases direct the action by altering the shape of the proteins and enabling them to do the necessary work.
Once there's a signal that sets off a series of responses, it's essential that, somewhere down the line, you can put the brakes on that signal and reset the system. "You have to introduce something that counteracts the original directions of the kinases," Perego adds. That's the job of the phosphatases, which are also enzymes.
In some bacterial cell reactions, it's easy for scientists to determine the signals. But others, like TV static, aren't clear at all—one of these is the reaction that initiates the development of the bacterium into a spore.
"This is one of the mysteries we're trying to solve. Why does a cell that grows, divides, and divides again decide at a certain point to stop and transform itself into a spore, something that isn't really living anymore?"
Tracking the Spoor of Sporulation
Human infection from Bacillus anthracis is a result of this bacteria's ability to outwit the body's immune system. The pathogenic process is set in motion when B. anthracis acquires some extra DNA, called a plasmid, which is a vector for the introduction of new genes into a bacterial cell. Plasmids allow B. anthracis, an otherwise harmless bacterium, to produce deadly toxins and to build a protective shell called a capsule.
The body's immune system does what it can: it easily destroys most of the spores that first infect a host. But a few spores escape and travel out from the lungs to the safe harbor of the lymph nodes or bloodstream. Once there, they start growing, each makes a protective capsule around itself, and then they can keep growing. In this protected state, the immune system is powerless, and the bacteria keep churning out toxin.
"This ability to escape the immune system, along with the fact that the cell's extra DNA is capable of making a toxin, is bad news," Perego explains. "Toxemia and bacteremia can result and the patient can die."
Scientists have known for some years that B. anthracis doesn't sporulate in the blood, and this fact was the starting point in Perego's research into this bactericum's mechanisms of infection. Her work has resulted in some surprises and the publication of two academic papers that shed light on this generative process.
"Our research has revealed that B. anthracis may have developed mechanisms that allow it to efficiently sporulate in the most favorable environments and to delay sporulation when sporulation would kill it," Perego says. "So B. anthracis is a very smart creature."
The ultimate opportunist, the bacterium doesn't sporulate in the blood of an infected patient, because if it did, it would be destroyed by the patient's immune system. However, when it finds itself in animal waste, it sporulates in order to survive.
Perego's next question was: what specific mechanisms allow it to do this?
Making the Plasmid Connection: The Roles of Pxo2 and Pxo1
Perego knew that, in addition to its chromosome, the ability of B. anthracis to cause disease is associated with its plasmid content. The two plasmids that come into play are called pXO1 and pXO2. The first contains the genes for the production of the toxin that damages the cells of the infected host; the second contains the genes for the production of the capsule, which protects the cells from the immune system. This toxin-capsule combination is what makes B. anthracis lethal.
"What we have found is that the two plasmids contribute to the virulence of the organism, but that it isn't this simple—there are more factors involved. One of these factors is a phosphatase that, if deregulated, does not allow B. anthracis to sporulate. If this bacterium were to sporulate, it could easily be destroyed by the immune system because it loses the ability to make the capsule and the toxin."
In order to be effectively virulent, B. anthracis has to be in the non-sporulating state: thus the phosphatase Perego's lab has characterized can ensure that the bacterium stays in this non-sporulating state. There's a major contingency, however: the phosphatase could be inhibited (blocked) by a small molecule called a pentapeptide (a chain of five amino acids).
This pentapeptide is produced by the B. anthracis cells themselves in the form of a bigger protein of 44 amino acids together with the phosphatase. However, while the phosphatase remains inside the cell, the 44 amino acid protein is exported outside the cell. There, it is processed down to the five amino acid pentapeptide that is reimported into the cell. This export-import business is a regulatory system to ensure that the pentapeptide inside the cell is of the appropriate concentration at any given time during the cell growth cycle.
"If the cells are growing in the bloodstream, most of the pentapeptide gets either degraded or diluted away. This means that very little is reimported into any given cell growing in the bloodstream. In turn, this means that the phosphatase does not get blocked and so the cell cannot sporulate."
However, in the free environment—in animal waste, for example—the pentapeptide does not get washed away, so it gets reimported in sufficient amount to block the phosphatases, allowing the cell to sporulate.
"This system of regulation is very convenient for the B. anthracis cell, because in order to survive and be an efficient pathogen, it better be able to sporulate in the environment, but not in the bloodstream of the infected host," Perego explains.
The second mechanism brought in by pXO1 and pXO2 that regulates the ability of B. anthracis to sporulate also has to do with inhibiting the sporulation process when the bacterium infects the host. This mechanism relies on two proteins (one encoded by the pXO1 plasmid, the other by the pXO2 plasmid) that are highly homologous to one kinase protein, which is encoded by a gene located on the chromosome. Perego underscores the importance of this kinase in the sporulation process of B. anthracis. Her lab found that the complication at this stage is that the two proteins encoded by the plasmids can inhibit the activity of the kinase and therefore block sporulation.
"We concluded that the two virulence plasmids each encode a protein that can promote the virulence of the organism. This is a new discovery about the regulatory mechanism controlling the activity of a bacterium kinase," she says.
Translating Basic Science into Real-World Practicality
Perego terms her work "an intellectual exercise," but adds that it may well have practical applications down the line.
One important question is whether an anthrax infection could be cured by inducing the cells in the infected human body to sporulate. If they sporulate, Perego reasons, they won't make the toxin anymore "so this bacterium could be destroyed by the immune system without a patient having to go through six months of therapy with antibiotics and their various side effects."
For now, Perego says she feels good about the discoveries she has made. "When things go right in the lab, especially after some weeks of disappointment, I somehow feel lighter. There's real joy in finding something I may have suspected, but can now prove.
"When we make discoveries like this, I feel—let me put this another way... No, that's it: I feel lighter."
Send comments to: mikaono[at]scripps.edu

Associate Professor Marta Perego says, "This is one of the mysteries we're trying to solve—why does a cell that grows, divides, and divides again decide at a certain point to stop and transform itself into a spore, something that isn't really living anymore?" Photo by Kevin Fung.
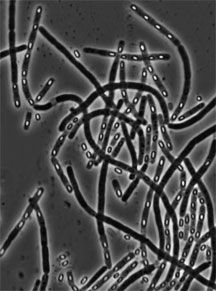
Marta Perego first came to Scripps Research to work in the lab of Professor James Hoch, where she studied the mechanisms of the bacterium Bacillus subtilis. Image by James Hoch.
