

The Front Part of the Cell
By Jason Socrates Bardi and Rachel Hanley
It's a sunny afternoon on a recent day in La Jolla, and Associate Professor Richard Klemke and Research Associate Yingchun Wang of The Scripps Research Institute are explaining the science that recently won Wang a $135,000 grant from the Susan G. Komen Breast Cancer Foundation.
The Komen Foundation is perhaps best known for its decades-long sponsorship of the Race for the Cure®, a series of five kilometer races that Nancy Brinker, the foundation's founder, started two decades ago. They have grown from a single race, run by 800 people in 1983, to about 100 different races held in various cities nationwide. About a million people are expected to run this year alone. San Diego will host the next Komen Race on Sunday, November 6, 2005.
About 25 percent of the money that will be raised in that San Diego event will support the national Komen Foundation's Award and Research Grant Program, which funds projects in breast cancer research, health education, breast cancer screening, and treatment around the world from the organization's headquarters in Texas. The remaining 75 percent remains with the foundation's San Diego affiliate where it will also be used to fund breast cancer research—much as this year's award to Scripps Research is being paid with money raised in past races.
Foundation support like this is important, says Klemke, because it provides crucial funds to support salaries for overseas scientists who are working in the United States.
"It is quite an honor," adds Wang, who was selected for the highly competitive award because he is applying new cutting-edge tools to cell metastasis, one of the most important areas of breast cancer research. Klemke, Wang, and their colleagues are involved in research on aspects of the basic biology behind metastasis—trying to identify the signal transduction mechanisms that lead to the spread of breast cancer cells.
Crawling Cells
Cancer occurs when normal cells acquire subtle mutations within their DNA. Often these mutations make the cancer cells resistant to normal programmed cell death, and the cells will divide over and over, forming a solid tumor. Also common to cancer cells are mutations that lead them to metastasize—a word that comes from a Latin construction meaning to change position.
Metastasis is a dangerous phenomenon in which cancer cells separate from the original tumor, move into the bloodstream or lymphatic system, anchor in a distant tissue or organ, and begin a new tumor. Although science and medicine have made tremendous strides in early detection and successful treatment, breast cancer and melanoma still claim tens of thousands of lives a year—usually the end result of metastasis. In fact, the U.S. Centers for Disease Control and Prevention (CDC) reported that an estimated 215,990 U.S. women were diagnosed with new cases of invasive breast cancer and tens of thousands of women died of the disease last year alone.
An obvious requirement for metastasis is motility, the ability of a cell to get up and go. "Migration is a fundamental ability that cells have," says Klemke, "[But] they need to be stimulated one way or another."
Often when a migratory cell moves, it is responding to a chemical stimuli—a gradient of molecules in the tissue or bloodstream surrounding the cell that it can detect and follow. This type of cell migration is called chemotaxis, and it happens in both healthy and diseased cells. In a typical immune response, for instance, inflammatory proteins called cytokines will be released by immune cells at a site of inflammation and then other immune cells will use these cytokines like a trail of breadcrumbs to home in on the site of infection and destroy the pathogens that are causing it.
Chemosensing is an active process, and for a cell to follow a chemical gradient, it must coordinate the activity of a swarm of protein "signals" that lead to the cell's movement. The process involves dozens, if not hundreds, of human genes. A cell has receptors around its outer membrane that sense external chemical cues. With swarms of these receptors on their surfaces, cells can sense changes in the concentration of chemicals and tell in which direction gradients point. These receptors then signal other proteins inside the cell to spread in the direction of the gradient. This signaling starts polymerization of actin, the structural protein inside the cell, causing the formation of a protrusion known as a "pseudopod" in the direction of the gradient, and blocks receptors on the tail end of the cell in what is known as global inhibition.
"Our goal is to decipher the signaling networks that regulate chemotaxis and to determine how they are altered in metastatic cancer cells," says Klemke.
Chemosensing and Cancer
What Klemke and Wang want to find are the key genes involved in breast cancer metastasis, and the Komen Foundation grant that Wang received should enable that.
Wang and Klemke will investigate chemotaxis related to the signaling of a protein called CXCR4, which is found on the outside of cancer cells. CXCR4 is a receptor that cancer cells use like antennae to feel their way from their parent tumor to distinct sites in the body where they will establish a new tumor. CXCR4 senses another molecule, called SDF-1α, and this chemosensing is the beginning of metastasis, leading to pseudopod formation and cell crawling.
But Wang and Klemke want to know the complete sequence of events that takes place when CXCR4 senses SDF-1α. Specifically, they are interested in those genes that are involved in tyrosine phosphorylation—the attachment of a phosphate group (a phosphorous surrounded by oxygen atoms) to distinct sites in protein chains where there is a tyrosine residue.
Phosphorylation is a key mechanism that cells use to bring proteins to bear. Instead of having to express proteins when they are needed and degrade them when they are not, phosphorylation can be used as a switch to turn a protein on or off, so to speak. The phosphate attached can determine factors such as whether a protein can cross a membrane, whether one protein will associate with another, or whether a protein is active.
This is a hot area of biomedical research because some of the proteins that are involved in phosphorylation and control some part of cell motility (and ultimately cancer metastasis) may be amenable to chemical control—the creation of new drugs that could block their action and slow or stop cancer metastasis.
"Most of the signals that people are working with and identifying right now are the most abundant signals—the main effector proteins," says Klemke. "Really, what we need to do is to identify the low-abundant proteins that are mediating the specific response." Despite the fact that the proteins are scarce, and hence difficult to study, they may still play a primary role in cancer metastasis.
In order to find these elusive effector proteins, Klemke and Wang are using a technique that Klemke has developed over the last decade for looking specifically at the leading edge of a migratory cell. By comparing the proteins that are in the front to the proteins that are in the rest of the cell, they hope to identify those few involved in metastasis.
Studying the Front Apart from the Rest
The technique essentially involves placing the cells in the top chamber of a two-chamber container with a concentration of chemokines like SDF-1α in the bottom chamber and a small hole in between. If a cell is doing what it should, it will sense the chemokines, move towards the source, and attempt to crawl through the hole. The hole is tiny, typically about three microns across, which is approximately the size of the opening through which a cell in the body will crawl.
"Then we can harvest the pseudopod and cell body separately," says Klemke. The cells can be fixed in methanol and everything but the pseudopod scraped off, and the proteins in the pseudopod can be identified using state-of-the-art proteomics and mass spectrometry techniques. "Yingchun has developed methods to analyze both low and high abundant phosphotyrosine proteins from the pseudopodia," says Klemke.
In collaboration with Scripps Research Professor John Yates, Wang is implementing a proteomics effort to identify the proteins. This effort employs a highly sensitive method called multidimensional protein identification technology, or "MudPIT," which relies on mass spectrometry and was originally developed by Yates and his Scripps Research colleagues. In MudPIT, a complex mixture of proteins and peptides are separated using 2-D liquid chromatography, and then the separate proteins are digested (chopped into smaller "peptide" pieces with enzymes) and subjected to the sensitive technique of mass spectrometry (which identifies the components based on their masses). The mass spectrometry instrument detects these masses and uses sophisticated software to identify individual proteins.
In the end what you get through a comparative process is a profile of the proteins unique to the front and back ends of a crawling cell. Klemke's laboratory has already identified several candidate proteins using this technique that may be key players in pseudopod formation and cell movement.
"Once we identify candidate proteins, we can manipulate and test them for their effect on cancer cell metastasis," says Klemke.
They start by using a technique known as RNA interference (RNAi), which is a natural defense mechanism cells employ to destroy certain types of viruses. A cell has a mechanism for destroying double stranded RNA, which it recognizes as viral in origin since the natural RNA inside a cell is single stranded. In destroying the RNA, a cell can prevent the viral replication of double-stranded RNA viruses.
The technique of RNAi hijacks this machinery to turn off normal cells. Basically it involves delivering small, 20-base pieces of double-stranded RNA into a cell that, once inside, will anneal to pieces of RNA that have complementary sequences. By designing the short pieces of RNA to match one particular gene, the intracellular response will specifically silence the expression of that gene.
Furthermore, once these proteins are identified, they can be selectively labeled with antibodies linked to green fluorescent protein (GFP) and directly observed in a cell.
Klemke's interests, and what he envisions to be the future of signal transduction research, is finding which of these signals are active—which are phosphorylated and dephosphorylated, for instance—during cancer cell metastasis.
"In the end, we hope that we can knock out the metastatic signals and thus block the spread of cancer in humans," says Klemke.
Send comments to: mikaono[at]scripps.edu

Scripps Research investigators Richard Klemke (left) and Yingchun Wang want to find the key genes involved in breast cancer metastasis.
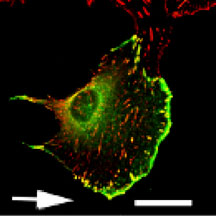
Deconvolution image of a migrating NIH 3T3 cell transfected with GFP Lasp that was fixed and stained with anti-vinculin antibodies. Note the colocalization (yellow) of focal adhesions (red) and GFP Lasp (green) at the leading front of the pseudopodium. Arrow indicates the direction of cell migration. Scale Bar = 28µm. Reproduced from The Journal of Cell Biology, May 10, 2004, Vol:165(3), pg. 421–fig.1 by copyright permission of The Rockefeller University Press.
