

How Do You Spell Recognition? C-O-R-E-C-E-P-T-O-R
By Jason Socrates Bardi
A group of scientists at The Scripps Research Institute has solved a mystery that has dogged immunologists for many years: how T-cell receptors interact with their coreceptor proteins at the beginning of an immune response.
T-cell receptors and coreceptor molecules are both large proteins displayed on the surface of T cells, and they play an essential role during the beginning of an immune response. T cells become activated after their T-cell receptor recognizes antigen—small pieces of foreign pathogens—on the surface of other cells. Coreceptors, while they do not recognize foreign antigen, also bind to molecules on neighboring cells and help in T cell activation.
Although the basic events and consequences of T cell activation have been understood for years, immunologists have long debated some of the specific details. One of the major points of contention has been over the nature of the interaction between the T cell receptor and coreceptor molecules. They both interact with the same target, but their interaction with each other has been hotly debated.
Some immunologists have suggested that T-cell receptors and coreceptors must come together before the T-cell receptor can sense antigen. Others have suggested that it is the sensing of antigen that brings them together. Now, in an article in the August issue of the journal Nature Immunology, Scripps Research Professor Nicholas Gascoigne, Research Associate Pia Yachi, a graduate of the institute's Kellogg School of Science and Technology, and their colleagues show that it is the latter—T-cell receptors and CD8 coreceptors are brought together during antigen sensing.
"They are actually pulled together during antigen recognition and are not close together before," says Gascoigne, who observed this with the technique of fluorescence resonance energy transfer (FRET), a sophisticated way of looking at the real-time interactions between molecules in living cells.
Gascoigne, Yachi and colleagues also show the importance of endogenous non-stimulatory peptides in the immune response. These are molecules presented simultaneously with the foreign antigens to which the T-cell receptor binds, and they increase the interaction of the T-cell receptors and coreceptors.
These findings are important because the recognition of antigen and activation of T cells is one of the most basic processes that takes place during an immune response, and the results described by Gascoigne, Yachi, and their colleagues help illuminate how it occurs. "It tells us something about how T cells can distinguish a very few antigenic proteins within a great sea of [molecules]," says Gascoigne.
Antigen Recognition and the Immune System
Over millions of years of evolution, the immune system has built up myriad ways of countering potentially lethal infections. In many organisms, including humans and other mammals, the adaptive immune response plays a critical role in ensuring our survival, by allowing the generation of numerous immune cell clones that are specifically designed to wipe out viruses, bacteria, and infected human cells.
The adaptive immune system must be kick-started by professional antigen-presenting cells, which take up foreign proteins and other pieces of bacteria or viruses and process them—chopping these "antigenic" components into pieces. The antigen-presenting cells then display the antigens on their surface within proteins called major histocompatibility complex (MHC) proteins. They also move into the local lymph nodes and show the antigen to naïve T cells, which periodically enter the lymph nodes as they circulate through the bloodstream.
T cells, so named because they are created in the thymus, are one of the major types of adaptive immune cells, and they come in two flavors, both of which are crucial. Helper T cells are essential players in the adaptive immune response that produce a swill of chemicals to clear the infection and activate other immune cells. Cytotoxic T lymphocytes—the other type of T—are also known as killer T cells because they go around destroying cells that are infected with the foreign pathogens. Being able to do so is crucial, because otherwise foreign pathogens such as viruses could replicate inside an infected cell.
Another crucial ability that killer T cells exercise is the ability to distinguish a cell that is infected with a pathogen from one that is not infected. T cells can do this because they carry a unique "T-cell receptor"—a protein that the cells display on their surface that binds to another specific structure. These T-cell receptors are also essential during T cell activation, the crucial first step of the adaptive immune response.
In this sense, the T cells in the body are a diverse population of millions of similar but distinct cells, each of which carries its own T-cell receptor. After T cells develop in the thymus, they circulate as inactive naïve cells until their T-cell receptor encounters a professional antigen-presenting cell with the right antigen loaded in its MHC. Once a circulating naïve T cell with the right T cell receptor sees this, it becomes activated, replicates over and over, and begins the adaptive immune response.
But the activation of a T cell is not like a simple flicking of a switch. The detailed picture of the process is much more complicated, requiring the coordinated activity of many different proteins besides the T-cell receptor. For instance, the T-cell receptor is connected to another nearby protein called CD3, and successful binding to MHC–antigen will cause the phosphorylation (the attaching of a phosphate group) of the CD3. This, in turn, causes other phosphorylation events in a network of other small proteins associated with the T-cell receptor, and this leads eventually to the activation of the T cell.
In fact, immunologists describe the interface between a T-cell and an antigen-presenting cell using a term they borrowed from the neuroscientists—synapse—to evoke the complex cellular communication processes focused around this area where the T-cell receptor and the MHC–antigen interact.
Coreceptors and FRET
Activation of a T cell is made even more complicated by the fact that there are a number of safety mechanisms that ensure T cells will only be activated when it's really necessary. If T cells didn't have these safety mechanisms, then they might become activated accidentally and wind up doing more damage to a body's tissues than a pathogen would.
One of these is the requirement that T-cell receptors be cross-linked by MHC–antigen complexes on the antigen-presenting cell for T cell activation to occur. This is not necessarily a tall order, since antigen presenting cells are covered with MHC and T cells are covered with T-cell receptors. But not all of the MHC will be loaded with the correct antigen—in fact most of them are loaded with normal, non-antigenic peptides. Therefore, the T cells need something to help them sort through the proverbial haystack to find those few needles and match up their T-cell receptors with the correct MHC–antigen. This is where they turn to a completely separate set of proteins called the coreceptors.
These coreceptors—including CD4, a coreceptor displayed by helper T cells and CD8, a coreceptor displayed by killer T cells—are essential for the activation of the T cell because they interact with MHC molecules and help the T-cell receptors recognize MHC–antigen. These interactions are known to regulate activation by determining how engaged a T cell is with a neighboring antigen-presenting cell.
Coreceptors like CD8 do not bind to the part of MHC that displays the peptide but instead bind to another part of the MHC molecule in a specific—but weak—interaction. Because of the specificity, CD8 is able to bind to MHC regardless of which antigen the MHC displays, and because the reaction is weak, CD8 and MHC can easily associate, dissociate, reassociate, and deassociate as the surfaces of a T cell and an antigen presenting cell meet.
Wanting to investigate this interaction in living cells—the subject of the scientific debate—Gascoigne and his colleagues turned to FRET, which works through the use of fluorescent molecules that will absorb and re-emit light of a certain wavelength. Under a microscope, cells containing these molecules fluoresce when illuminated with light of the correct wavelength. By attaching a cyan fluorescent protein to one end of the T-cell receptor and a yellow fluorescent protein to the end of the CD8 molecule, the scientists could observe two different types of fluorescence. Normally cyan fluoresces cyan—a pale blue—but when the cyan fluorescent protein is close to the yellow fluorescent protein, the cyan transfers its energy to the yellow, and yellow fluorescence is observed.
Now, Gascoigne and his colleagues describe in an article in their Nature Immunology article how FRET reveals the interaction of the CD8 coreceptor with the T cell receptor during recognition of MHC–antigen. They show that the molecules are not very close together on the surface of the living cell, but when a T cell is engaging an antigen-presenting cell, the binding of CD8 to MHC complexes helps the T cell recognition by concentrating MHC molecules.
Significantly, Gascoigne and his colleagues showed that the key lies in a general stimulation of the T cell with "non-stimulatory" peptides. When there is just a small amount of MHC on the cell surface, there is very little stimulation of the T cells, even though all of the MHC molecules are loaded with the exact antigens the T cells recognize. Without the presence of the non-stimulatory peptides, the ability of T cells to recognize the antigen-presenting cells is greatly reduced.
In other words, the more the T cell engages with the antigen-presenting cell in a general sense, the more likely its T-cell receptor will engage the correct MHC–antigen in a specific sense.
The article, "Non-stimulatory peptides contribute to antigen induced CD8-TCR interaction at the immunological synapse" by Pia P. Yachi, Jeanette Ampudia, Nicholas R.J. Gascoigne, and Tomasz Zal appears in the August issue of Nature Immunology. See: http://www.nature.com/ni.
This work was supported by the National Institutes of Health.
Send comments to: jasonb@scripps.edu

Professor Nicholas Gascoigne and Research Associate Pia Yachi have published an article in the August issue of the journal Nature Immunology.
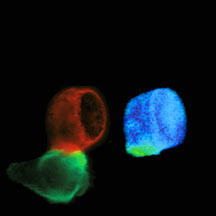
These images of a T cell contacting an antigen presenting cell were taken with a fluorescence microscope. The left image shows the T cell in red and the antigen presenting cell in green. The right image shows that T cell receptor and co-receptor CD8 molecules are interacting in the interface—the immunological synapse—between the T cell and the antigen presenting cell. The interaction was measured by FRET, shown as the green and yellow regions of the image.
