

Speed and Detection
By Jason Socrates Bardi
Last week, Scripps Florida Professor John Hogenesch was on the telephone talking about how what is old is new again.
He is returning home to Florida, where he moved with his family last December as the head of Genome Technology at Scripps Florida. Hogenesch grew up in Gainesville in the 1970s, and moved to the West Coast in the late 1980s to attend college at the University of Southern California. From there, he moved "back East"—halfway—and spent most of the 1990s in Chicago, getting his Ph.D. in neuroscience from Northwestern University.Then he moved to La Jolla for postdoctoral training and joined the laboratory of Professor Steve Kay at The Scripps Research Institute, with a joint appointment at the Genomics Institute of the Novartis Research Foundation (GNF), also in La Jolla.
Now, he returns to his old state of Florida to do something brand new. Hogenesch is setting up the Cell-Based Screening Program at Scripps Florida's temporary facilities in Jupiter.
A Gene Atlas
Hogenesch's move is a return to familiar territory in more ways than one. When he was with GNF, he did a lot of work using what are known as gene chips to determine which genes from the human genome are expressed in various tissues.
These "expression profile" experiments involve taking cells from the particular tissues, recovering the cells' expressed genes (in the form of messenger RNA, or mRNA), chopping the mRNA into fragments, and plopping the mixture of fragmented mRNA on a gene chip—a glass or silicon wafer that has thousands of short pieces of DNA attached to it with sequences corresponding to known genes. These short pieces are laid out in a grid, and genes that are expressed in the tissue will bind to complementary pieces of mRNA on the grid. Then, by looking to see which pieces on the grid have RNA bound to them, the scientists are able to determine which genes were expressed in the sample.
DNA and RNA chips have become a standard tool for genomics research in the last decade, and scientists can easily put a large number of different oligonucleotide probes—even all the known genes in an organism such as mouse or human—on a single chip.
Using this technology, Hogenesch and his GNF colleagues created a Gene Atlas by taking some 36,000 genes in the human and mouse genomes and looking at their expression across a variety of 80 different tissues in the body. They were not selective in choosing the genes. Instead, they used every gene they could get their hands on, known or predicted from the genome project.
"If it looked like a gene, we looked at it," says Hogenesch, who calls this "unbiased" screening. "It was a whole different experiment on a whole different scale."
While describing this to me over the phone, Hogenesch directed me to the web site that was produced from this effort, and he asked me to enter a favorite gene or protein. The first that came to mind was CCR5, the human receptor protein that human immunodeficiency virus binds to, so I typed that in.
Down my screen unfolded a bar graph with a list of different tissues types. On the top right corner was a box with all the different aliases by which CCR5 has been known. The screen showed only a few tissues contained CCR5 in high levels. Whole blood was one, and a couple of different immune cells that can be found in the bloodstream were there as well. I realized at once that this was exactly what one would expect. HIV uses this receptor protein to gain entry and infect cells in the bloodstream, so those tissues are the ones in which you would expect to find CCR5.
The purpose this database, says Hogenesch, was to help academic researchers make connections between where a gene is expressed, what other genes are being expressed there, and what their physiological functions might be.
Biology in Broad Strokes
RNA expression can tell you where in the body a certain gene is expressed, and from this you can infer the gene's functions. However, scientists would like to probe a gene's function more directly by turning them on and off and looking at the consequences in a live cell.
At Scripps Florida, Hogenesch and his colleagues intend to go even further than finding gene expression. They now want to develop and implement technologies to focus on the next level of information—not just where genes are being expressed but which of the expressed genes are actually functional.
To do this, they are creating a system for turning on and off tens of thousands of different genes in a particular tissue and looking at the effect on the cell. They have tens of thousands of what are known as plasmids—large circular pieces of DNA that contain a gene that can be turned on individually in an experiment. With an array of these plasmids arranged inside tiny wells on a plate, they can place cells from a particular tissue in each well and turn on the genes in the plasmids. Similarly, they have been making systems to do the inverse—specifically silencing or shutting off particular genes inside cells.
To do this, they are employing a new technology known as RNA interference (RNAi), which is a natural defense mechanism cells employ to destroy certain types of viruses. Cells have a mechanism for destroying double stranded RNA, which it recognizes as viral in origin since the natural RNA inside a cell is single stranded. In destroying the RNA, a cell can prevent the viral replication of double-stranded RNA viruses.
The technique of RNAi hijacks this machinery to turn off normal cells. Basically it involves delivering small, 20-base pieces of double-stranded RNA into a cell that, once inside, will anneal to pieces of RNA that have complementary sequences. By designing the short pieces of RNA to match one particular gene, the intracellular response will specifically silence the expression of that gene.
As part of the cell-based screening program, Hogenesch and his colleagues have taken about 7,000 genes that are either known or suspected to be involved in aspects of human health and disease and can be targeted therapeutically—genes that encode proteins like G-protein coupled receptors, phosphatases, kinases, and cell surface proteins. For each of these 7,000 targets, the scientists have created four different small RNAs (since RNA interference for a particular sequence only works 50 to 70 percent of the time), and with an array of these RNAi arranged inside tiny wells on a plate, they can place cells from a particular tissue in each well and turn off particular genes.
"This is going to point out which of the remaining genes in the genome play a really important functional role," says Hogenesch. "When you turn the genes on or off in a cell, that gets at causality—it's an exciting way to do biology."
With cell-based screening assays, he and his colleagues will try to determine a baseline of annotation of gene function as well as providing useful chemical probes for understanding important cellular pathways. These technologies will be integrated with other technologies such as informatics, RNA expression dynamics, genetics, and proteomics to select promising disease or pathway targets for further study. The scientists will also look at the effect of exposing whole cells to small, drug-like molecules that target these genes and proteins.
One of the applications of this approach will be geared towards understanding the biology of circadian rhythms.
Body Clocks
Circadian rhythms are the cyclic changes in the expression of genes in the body. Humans, mice, and other organisms have these internal rhythms so that they can use their bodies as "clocks" to keep track of time and coordinate biological processes to the rhythm of day and night.
These circadian rhythms offer distinct advantages to organisms that use them. Plants, for example, shut down photosynthesis at night, and they gear up their photosynthetic machinery and raise their leaves just before dawn. They use their clocks to measure day length and in that way anticipate changes in the seasons—a system that determines when they shed their leaves, produce seeds, and make flowers or fruit.
Humans also have circadian rhythms, and we entrain our internal clocks to the 24-hour day. Under normal conditions, we time our major activities with daylight, we sleep during the nighttime, and some of our vital signs follow this pattern. For instance, our blood pressure fluctuates daily, rising and falling at predictable times of the day or night.
But knowing that we have a body clock is not the same as knowing how our clock works. In the last few years, Hogenesch and his colleagues have turned their research efforts to discover the molecules and pathways that lie behind circadian rhythms. For example, scientists realized that our bodies set their clocks through receptors that detect light, but they did not know what the molecular components of this clock were.
Until recently, scientists had speculated that the key receptor would be a protein called rhodopsin, which captures light in the retina as part of the mechanism of normal vision.
The retina is that red venous tissue at the back of the eye that's visible whenever we take a picture of somebody without using red-eye reduction, and it is where the detection of light takes place. Rhodopsin can be found on the surface of specialized photoreceptor cells in the retina known as rods and cones. With rhodopsin, these rods and cones detect the light that enters through the pupil and transmit electrical impulses to nerve cells, which carry the information to the brain. So if rhodopsin was present on rods and cones and ultimately responsible for vision, scientists reasoned, then perhaps it was also involved in detection of light.
However, there was good evidence that the mysterious protein was not rhodopsin. For one thing, in 1988 a team of researchers in Japan discovered that mice that are born blind but with intact retinas can still sense light through their eyes. They cannot see, but they still wake up in the morning when it is light and go to sleep at night when it is dark. This is true even if you simulate suddenly shifting them into another time zone with artificial light. If you move morning and night back by several hours, the blind mice will eventually adjust their schedules to awake and go to sleep with the shifted light.
Likewise, certain people who were born blind because they lacked rods and cones could still maintain their circadian balance. However, humans and mice with no retinas cannot sense light at all and cannot maintain circadian rhythms. All this suggested a few years ago that some other light receptor in the mammalian retina senses light, but not for seeing—a non-visual photoreceptor.
After Hogenesch came to La Jolla, he became part of a team of scientists led by Kay that was looking to determine the identity of this mysterious non-visual photoreceptor. And in the last several years, they have focused on one protein—called melanopsin—that is expressed on the surface of a type of cell known as an intrinsically photosensitive retinal ganglion cell.
"It's the key player in the photo receptor system," says Hogenesch of melanopsin. These light receptors played no part in vision, but they take in light and communicate to the brain that the light is there.
A few years ago, Kay and Hogenesch published a paper in the journal Science in which they showed that melanopsin was required for the functioning of the clock. In addition to Kay and Hogenesch, authors on the paper included Satchidananda Panda, a graduate of Scripps Research's Kellogg School of Science and Technology and formerly a postdoctoral fellow at GNF, and Trey Sato, who was formerly a postdoctoral fellow at the Scripps Research La Jolla campus and is now a staff scientist at Scripps Florida.
Recently, another paper appeared in the journal Science that showed melanopsin is sufficient for the functioning of the clock. Hogenesch was a coauthor of this paper with Panda, who is now at the Salk Institute for Biological Studies. The paper also showed that melanopsin signaling was coupled to several other signaling proteins, including a type of transient-receeptor potential (TRP) channel called TRPC3.
This is a robust story on melanopsin, says Hogenesch, but not a complete one. While Panda will continue working on this story at the Salk, Hogenesch and his colleagues will continue working to screen thousands of human genes and try to determine which ones are involved in the generation of circadian rhythms.
"We're pretty sure we can narrow this down to dozens instead of thousands of genes," says Hogenesch. "This is going to make it a lot easier for us to determine what are the key players.
Send comments to: jasonb@scripps.edu

"When you turn the genes on or off in a cell, that gets at causality—it’s an exciting way to do biology," says Professor John Hogenesch.
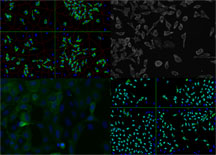
With cell-based screening assays, Professor John Hogenesch and his colleagues will try to determine a baseline of annotation of gene function as well as providing useful chemical probes for understanding important cellular pathways. Images from Beckman Coulter IC100 Image Cytometer, analyzed using Cytoshop. Click to Enlarge
