

Sweet Recognition
By Jason Socrates Bardi
At the March meeting of the American Chemical Society (ACS) in San Diego, I happened to run into Professor Chi-Huey Wong on my way from a lecture to a press reception. It was a busy month for Wong, who holds the Ernest W. Hahn Professor and Chair in Chemistry and is an investigator in the Skaggs Institute for Chemical Biology at The Scripps Research Institute.
That evening he was being presented with the 2005 ACS Award for Creative Work in Synthetic Organic Chemistry, which is sponsored by Aldrich Chemical Company, Inc. The next day, he told me, he would be delivering the award address at a symposium organized in his honor.
He would speak about carbohydrate synthesis, carbohydrate arrays, and how protein glycosylation (the attachment of carbohydrates to proteins) is the most important type of post-translational modification that we know of. Wong is one of the pioneers the field of oligosaccharide synthesis—the production of the type of complex sugars (or carbohydrates) that are commonly found attached to proteins and fat molecules in the human body. Wong has spent years designing systems for the synthesis of oligosaccharides and evaluating how these sugars are involved in interactions relevant to health and disease.
Carbohydrate structures are part of the language of life. They are like the accents on spoken words—they change the meaning without changing the spelling. Some even call carbohydrates the third alphabet, behind DNA and proteins. Though they are not charged with storing genetic information like DNA or acting as enzymatic workhorses like proteins, carbohydrates nevertheless do carry information and are responsible for important biological functions, playing a central role in many types of intercellular communication events, particularly in the immune system—roles that make many carbohydrates attractive targets for drug design.
The lion's share of such drug research has involved the interactions of small molecules with proteins or nucleic acids. Carbohydrate recognition, which surely has important biological activity given the volume of carbohydrates in the body, has lagged behind the fields of protein and nucleic acid recognition. One of the main problems is that carbohydrates are often so complex that they are among the most difficult molecules for organic chemists to synthesize. The work that Wong has done throughout his career is helping to change that.
More than fifty percent of the proteins in the human body are glycosylated, Wong says to the crowd gathered in one of the conference center's massive ballrooms. Most cells are covered with glycosylated proteins many of which have unknown functions. "[Finding] the functional aspects of carbohydrates is going to be the future," Wong says.
A few days after the symposium, I got an email from the journal Nature indicating that it was about to publish a paper on which Wong was a coauthor with a team of researchers including Mitchell Kronenberg and his group at the La Jolla Institute for Allergy and Immunology and David Ho at the Aaron Diamond AIDS Research Center and New York University. A few weeks earlier, another paper had come out by the same team in the Proceedings of the National Academy of Sciences. Kronenberg was the corresponding author on the former paper, and Wong was the corresponding author on the latter.
The research was on an important type of oligosaccharide-linked lipid that is recognized by a type of human receptor called CD1d—a recognition event that is a crucial part of the immune system.
Recognizing Bacteria
CD1d belongs to a family of receptor proteins (designated CD1a-d) that are present on the surface of two types of immune system cells known as Langerhans and dendritic cells. These are professional antigen presenting cells that play an important role in innate immunity. CD1 receptor recognition is vital for defense against common bacteria because Langerhans and dendritic cells use these molecules to "present" an immune cell known as a natural killer (NK) T cell with specific glyco-lipid antigens (fatty molecules that are components of the bacteria).
This presentation activates the NK T cells, and the NK T cells help to activate other components of the immune system and defeat the infection.
Once the NK T cells become activated, they expand in number and unleash a torrent of action aimed at clearing the infectious agent. They begin to secrete a large amount of two proteins—interferon-gamma, which activates macrophages, and interleukin-4, which activates helper T cells. The macrophages and the helper T cells then carry on the immune response and ultimately deal with the pathogens.
NK T cells are unusual in that they fall somewhere between innate and adaptive immunity. These cells arise in the thymus, and as mature cells, they stimulate an adaptive immune response and regulate a range of disease states, including diabetes, cancer, and pathogenic infections. Evidence for the cell's role in cancer can be seen in studies of mutant mice that are lacking NK T cells. These mice have a higher incidence of cancer, but if the deficient mice are transplanted with NK T cells, their prognosis much improves.
Like other T cells, NK T cells express T cell receptors (TCR)—although without the normal antigenic variability. However, NK T cells vary from other T cells lines in that they also express the "NK" receptors, which are designed to detect some of the lipids that many bacteria display on their outer surface by recognizing the CD1 receptor on the surface of the aforementioned Langerhans and dendritic cells.
Because they are one of the fundamental molecules through which the immune system recognizes the pathogenic world, CD1 receptors are a hot topic of interest in biology right now. Several groups at Scripps Research focus on CD1(see related stories on http://www.scripps.edu/newsandviews/i_20050321/wilson.html and http://www.scripps.edu/newsandviews/i_20041115/teyton.html.)
The First Natural CD1d Antigen
About 10 years ago, a group of scientists at Harvard Medical School identified the first antigen bound by CD1, but it was a glycolipid from a marine sponge and so probably not the sort of antigen that the human immune system normally encounters.
Since then, several other antigens that bind to forms of CD1 have been identified, including both natural human lipids and those from the cell walls of common bacteria, such as Mycobacterium tuberculosis and Nisseria gonorrheae.
Even so, scientists have not been able to identify any of the natural antigens bound by CD1d—despite the fact that people have been looking for several years.
The latest results by Wong and his colleagues appeared in two recent articles describing, for the first time ever, bacterial antigen for the CD1d molecule.
In the February, 2005 issue of the Proceedings of the National Academy of Sciences, described how Wong and graduate student Douglass Wu synthesized a bacterial glycolipid and showed that it could activate mouse and human NK T cells in vitro. The bacterial glycolipid, which technically is called an "alpha-galacturonosyl-ceramide," comes from a type of rod-shaped bacteria known as Sphingomonas wittichii, which are highly abundant in the environment.
Then, in an article that appeared in the journal Nature in March, Wong's collaborator Mitchell Kronenberg of the La Jolla Institute for Allergy and Immunology showed that mouse and human NK T cells also recognized and responded to the bacterial glycolipid in vivo. They found that the Sphingomonas glycolipid, when presented to mice as an antigen, generated NK T cells that killed the bacteria.
"It's important to identify the real ligands that trigger NK T cell activation, and now we are getting some," says Wong. "The next step is to see how the glycolipid is presented to the NK T cell. [That] could lead to the design of an antigen."
Such an antigen, says Wong, would be an interesting starting point for the future design of vaccines. Since activated NK T cells stimulate the production of interferon gamma, a pro-inflammatory protein that stimulates macrophages, helper T cells, and other immune system cells, molecules like the Sphingomonas glycolipids that activate NK T cells can be used as adjuvants in vaccines—additives that stimulate a non-specific immune response and boost the effectiveness of a vaccine against a specific pathogen like HIV or Plasmodium falciparum, the malaria pathogen.
And since NK T cells are implicated in a wide variety of non-pathogenic diseases such as autoimmune diseases and cancer, such antigens could also provide a key component of a new therapy for these conditions as well.
To read the article, "Recognition of bacterial glycosphingolipids by natural killer T cells" by Yuki Kinjo, Douglass Wu, Gisen Kim, Guo-Wen Xing, Michael A. Poles, David D. Ho, Moriya Tsuji, Kazuyoshi Kawahara, Chi-Huey Wong, and Mitchell Kronenberg, see the March 24, 2005 issue of the journal Nature (434, 520-525) or go to: http://dx.doi.org/10.1038/nature03407.
To read the article, "Bacterial glycolipids and analogs as antigens for CD1d-restricted NKT cells" by Douglass Wu, Guo-Wen Xing, Michael A. Poles, Amir Horowitz, Yuki Kinjo, Barbara Sullivan, Vera Bodmer-Narkevitch, Oliver Plettenburg, Mitchell Kronenberg, Moriya Tsuji, David D. Ho, and Chi-Huey Wong, see the February 1, 2005 issue of the journal Proceedings of the National Academy of Sciences (102, 1351-1356) or go to: http://dx.doi.org/10.1073/pnas.0408696102.
Send comments to: jasonb@scripps.edu

"[Finding] the functional aspects of carbohydrates is going to be the future," says Professor Chi-Huey Wong. Photo by Jason S. Bardi.
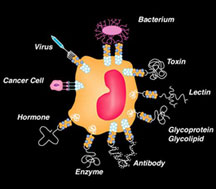
Carbohydrates and glycoproteins found on the surface of cells are often involved in molecular recognition. Click to enlarge
