

Aneuploidy Matters
By Jason Socrates Bardi
People who have parented a baby in California in recent years will recall the disquieting dread they felt at that point in the pregnancy where they were plagued by that chronic first-time parent concern: is my baby OK?
For me, it came around week 18 of my wife's pregnancy, when she had to submit a blood sample for California's so-called triple screen. I imagine that parents like me await the results of this screen with no shortage of anxiety because a positive result, while relatively rare, may be evidence of future developmental problems for their children.
California's triple screen measures the levels of two hormones and a protein in the blood. These levels can be affected by certain fetal abnormalities, such as massive genetic alterations within the developing fetus's brain cells. One such alteration is "aneuploidy"—where cells completely gain or lose whole chromosomes, the DNA cassettes that together contain all a cell's genetic information.
Aneuploid cells may have a profound impact on human health, which is one of the reasons why California's triple screen is designed to detect different types of aneuploidy. Having an extra copy of chromosome 18—a condition called Trisomy 18—can cause severe mental retardation and usually results in death at birth. Having an extra copy of chromosome 21—Trisomy 21—is a marker for Down's Syndrome, the most common cause of mental retardation.
Aneuploidy is also a well-known phenomenon in cancer, and scientists have long observed that many types of cancer cells, including most of the cancers that cause brain tumors, are aneuploid.
But remarkably, for all its infamy in cancer and developmental conditions, some instances of aneuploidy may not be so bad.
For the last few years, Professor Jerold Chun, who is an investigator in the Department of Molecular Biology and at the Helen L. Dorris Child and Adolescent Neuropsychiatric Disorder Institute at The Scripps Research Institute, has been studying aneuploidy in normal and diseased brains.
Now Chun, Scripps Research Associates Marcy A. Kingsbury and Stevens K. Rehen, and their colleagues have published two papers in as many months that suggest aneuploidy may in fact be part of the normal functioning of the brain.
Aneuploidy in the Brain
Chromosomes are the giant cassettes of genes that together make up the sum total of all the DNA in a cell. They are unique in number and composition for any given species, and they are usually the same for cells within one organism. Normal human cells have 46 chromosomes, for instance, whereas mice have 40. This unique chromosome composition is one factor that makes species unique. It is why, for instance, one cannot sexually cross animals of two different species with one another to make a viable new species.
But chromosome composition can also vary between cells in one organism—such as in aneuploid cells in the brain.
About a trillion cells reside in the average human brain, including around 10 billion neurons. Each of these cells got to where it is as the result of numerous cell divisions, differentiation, and migrations that transformed the most pluripotent stem cells in the embryo into one of the most specialized and most highly connected cells in the body.
Sometimes, an event takes place during this developmental history that leaves its indelible mark on a cell, making it aneuploid. Such events occur during normal cell division, when one parent cell duplicates all its DNA so that it has two identical sets of chromosomes. During the division, these sets are segregated to opposite ends of the dividing cell, and as division proceeds, the parent cell pinches apart at the middle so that it forms two daughter cells with identical copies of its chromosomes.
Aneuploid cells arise due towhat is known as a chromosomal missegregation. For reasons that are not entirely understood, a dividing cell sometimes separates its chromosomes unevenly so that its two daughter cells suffer a complete loss or gain of one or more chromosomes. Instead of the normal 46 chromosomes, an aneuploid cell might have 45 or 47.
The easiest way to understand how profound an effect on human health losing or gaining an entire chromosome can have is to consider how much DNA a cell loses or gains. If a gene were a song and a genome were a complete music collection, then a chromosome would be like a large set of related CDs in the collection. Losing an entire chromosome is not like losing a single Paul Butterfield CD. It's more like losing all the Beatles, Who, Cream, Rolling Stones, and Jimi Hendrix albums in the collection all at once—a whole generation's music gone from the genome.
There are many examples of diseases caused by the loss of a single gene from among the tens of thousands in the human genome. And if the loss of one gene is enough to cause disease, it is no wonder that aneuploidy causes disease—where thousands of genes can be gained or lost in one fell swoop.
But in recent years, scientists like Chun have been looking at the possibility that these chromosomal losses or gains might be one of the normal organizational principles that controls gene expression within neurons.
"[Aneuploidy] is a normal part of our brains," says Chun. "You have a range of ploidy in [normal] human neurons."
Aneuploidy Could Be Behind the Beautiful Mind
In the first of their two recent papers, Chun and his colleagues took samples of human brain tissue from tissue banks supported by the National Institutes of Health and maintained at Harvard University and the University of Maryland. These banks have brain tissue samples taken postmortem from thousands of organ donors.
In an issue of the Journal of Neuroscience last month, Chun, Rehen, and their colleagues examined chromosome 21 in the cells from the brains of a range of donors of various ages and states of health, and they discovered widespread examples of aneuploidy in neurons as well as other types of cells in the brain. Chun and Rehen also examined brain tissue samples taken from mice.
What they discovered was that around three percent of all the neurons and non-neuronal cells they looked at showed Chromosome 21 aneuploidy. This was true even in the brains of healthy individuals and individuals from all age ranges, and it suggests that aneuploidy is a widespread feature of humans as well as mice and other organisms.
However, a still unanswered question was what those aneuploid cells were doing in the brain. One possibility was the aneuploid cells are not functional—they are just there.
But Chun and his colleagues have recently shown that aneuploid neurons do function. In an upcoming issue of the Proceedings of the National Academy of Sciences, Chun, Kingsbury, and their colleagues provide the first evidence that aneuploid neurons are part of the neural circuity by showing that these neurons are connected to other neurons through standard synaptic wiring. Furthermore, Chun and Kingsbury demonstrate that the aneuploid neurons are functional by observing that they express what are known as intermediate early genes—a standard measure of neuronal function.
This sort of "constitutive" aneuploidy probably produces an enormous effect on a person's brain, and these results, taken together, raise a number of interesting questions about the role aneuploidy. Aneuploidy and the resultant differences in the genomes of various cells, says Chun, might play as much a role in human health as it does in disease. Controlling chromosome composition may be one not-so-subtle but nevertheless normal way the human body controls gene expression and neurotransmission. After all, even subtle changes in the expression of a single gene can affect the output of a neuron, and if you gain or lose an entire chromosome—two or three percent of your DNA—you have potentially acquired or lost a thousand genes, not just one.
If this alteration occurs during the development of a neuron, then it could potentially have effects over a person's entire life. Such changes might explain why diseases of the brain manifest so differently in different individuals, and why so many diseases of the brain are sporadic, and cannot be traced to a single gene. It also might explain why many psychiatric diseases are so hard to model—since different species have differing numbers of chromosomes and therefore radically different responses to aneuploidy.
And constitutive aneuploidy may also account for some of the underlying psychology that makes each individual unique: why some medicines such as antidepressants work for some people but not for others; why identical twins may be genetically identical but may vary drastically in terms of behavior; why one person might develop severe schizophrenia and not his/her twin; and why some people can live through an earthquake and be perfectly fine while others suffer from post-traumatic stress disorder after the event. And it also may provide hints about the genius question. How are geniuses made? Where does genius come from?
These are the sort of questions that neuroscientists like Chun and his colleagues are eager to pursue.
To read the article, "Constitutional Aneuploidy in the Normal Human Brain" by Stevens K. Rehen, Yun C. Yung, Matthew P. McCreight, Dhruv Kaushal, Amy H. Yang, Beatriz S. V. Almeida, Marcy A. Kingsbury, Kátia M. S. Cabral, Michael J. McConnell, Brigitte Anliker, Marisa Fontanoz, and Jerold Chun, see the March 2, 2005 issue of the Journal of Neuroscience (pp. 2176 –2180) or go to: http://dx.doi.org/10.1523/JNEUROSCI.4560-04.2005.
The article, "Aneuploid neurons are functionally active and integrated into brain circuitry" by M. A. Kingsbury, B. Friedman, M. J. McConnell, S. K. Rehen, A. H. Yang, D. Kaushal, and J. Chun, is being published by the journal Proceedings of the National Academy of Sciences and can be found on the "PNAS Early Edition" web site. See: http://dx.doi.org/10.1073/pnas.0408171102. The article will appear in a print version of PNAS later this year.
This work was supported by the National Institute of Mental Health, by Scripps Research's Helen L. Dorris Child and Adolescent Neuropsychiatric Disorder Institute, and by a National Science Foundation POWER grant. Additional support was provided through training fellowships by the Human Frontiers Science Program, the National Institute of General Medical Sciences, the PEW Latin American Program in Biomedical Sciences, the Swiss National Science Foundation, and the Pharmaceutical Research and Manufacturers of America Foundation.
Send comments to: jasonb@scripps.edu.

"[Aneuploidy] is a normal part of our brains," says Professor Jerold Chun. "You have a range of ploidy in [normal] human neurons." Here, Chun (left) stands with lab members: (left to right) graduate students Yun Yung, Beatriz Almeida, Research Associates Marcy Kingsbury (seated), Stevens Rehen, and Brigitte Anliker. Photo by Kevin Fung.
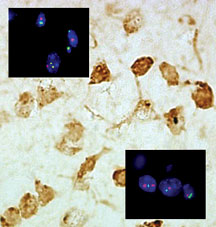
Functionally connected aneuploid neurons. Click to enlarge.
