

Good Molecules Gone Bad:
When Interferons Interfere with the Wrong Thing
By Jason Socrates Bardi
Ask any four people to give you the correct definition of irony, and you are likely to get four different answers, perhaps all equally correct. That’s because every field has its own examples of irony—from dramatic irony, where the intended meaning of a line is the opposite of its literal meaning, to political irony, where a bill passed into law accomplishes the exact the opposite of what was intended.
Now scientists at The Scripps Research Institute are describing a situation that can perhaps be called “immunological irony,” in which an immune system molecule causes immunosupression.
In this month’s issue of the journal Immunity, Scripps Research Professor Michael B. A. Oldstone along with his colleagues Bumsuk Hahm, Matt Trifilo, and Elina Zuniga report that certain viruses have the ability to subvert a class of antiviral molecules called type I interferons, an important part of our innate resistance to viruses. Type I interferons normally are produced early on in a viral infection and help the body clear the infection by inhibiting viral replication and by kick-starting other parts of the immune system.
“Interferon has always been considered a protective molecule—a good molecule [produced] to help the host,” says Oldstone. But, he adds, the protective effect of type I interferons depends on the state of the cells' differentiation in which they are produced.
If type I interferons are produced within the mature form of a kind of immune cell known as dendritic cells, they interfere with viral replication and are good for the host and bad for the virus. However, if the interferons are produced within a dendritic precursor cell, they actually shut down the expansion and development of mature dendritic cells, which is bad for the host because it suppresses a vital part of the innate and adoptive immune system and thus helps the virus.
Oldstone and his colleagues report that they observed this immunosuppression of dendritic cell precursors in vitro and in vivo when the cells were infected with two separate viruses—measles and a rodent virus called lymphocytic choriomeningitis virus.
These findings represent a major advance in our understanding of how some viruses interact with their animal hosts and suggest a new signaling pathway that might yield new targets for pharmaceutical intervention.
The Persistence of Viruses
Measles, which causes a maculopapular rash, fever, diarrhea, and occasionally death, is the most contagious infectious agent known to humankind, with an infection rate of 98 to 99 percent. Before a commercially available vaccine for measles came into use in 1963, and before the subsequent advent of mandatory vaccination programs in the United States, an estimated three to four million domestic cases occurred annually, and some 90 percent of the U.S. population had been infected with the virus by the age of 15. Measles is still endemic in tropical climates, such as southern Asia and sub-Saharan Africa, and the U.S. Centers for Disease Control and Prevention estimates that there are still an estimated 30 million cases of measles worldwide each year.
Lymphocytic choriomeningitis virus, a rodent virus discovered in 1933, has been used for decades by scientists and has contributed to our understanding of many of the basic concepts in immunology and virology. It belongs to the arenaviridae family—a nasty group of viruses that cause chronic, smouldering infections in rodents and acute, deadly outbreaks of disease such as Argentine hemorrhagic fever and Lassa fever in humans.
Oldstone has studied the measles and lymphocytic choriomeningitis viruses for a number of years, because they are excellent tools for examining two of the major problems with viral infections: persistence and immunosuppression.
Viral persistence is one of several possible outcomes of a viral infection, and not a particularly nice one for the host. Persistent viral infections can last years or even endure for the lifetime of the host organism, and the animal infected with the virus may have specific dysfunctions as a result. If the virus lives in the nerve cells, for instance, the learning functions of the animal can be impaired. If the virus replicates in the immune cells, the functioning of the immune system can be impaired.
When Oldstone began studying viruses a few years ago, the prevalent theory was that when viruses persisted, they did so because the host organism mounted no host response at all to the infection. But, as he determined over the course of several years of research, the host does make an immune response, but the response is not sufficient to clear the infection—often because the virus evades or suppresses the host immune system. Further, he and his colleagues showed how viruses could infect cells of the immune system, including CD4+ T cells, and cause immunosuppression—a decade before HIV was uncovered and shown to do the same thing.
Oldstone has made a career of studying host-virus interactions and his work has been recognized with numerous prizes, including the J. Allyn Taylor International Prize in Medicine and the Biomedical Science Award from the Karolinska. Now he and his group have found evidence that one of the ways viruses prevail is by turning the immune system against itself. Viruses such as measles and lymphocytic choriomeningitis virus suppress the immune response with type I interferons—which should be suppressing them.
Immune Activation and Suppression in One Molecule
Immunosuppression is a major problem in viral infections because it can make an otherwise not-so-lethal infection lethal. Only about one out of every million cases of measles, for instance, is lethal because of the action of the virus. (These are cases where measles infects the brain and the body's immune system makes a prolific response and causes massive damage to the central nervous system.) However, fatality rates in measles can be quite high because of virus-induced immunosuppression leading to secondary bacterial or parasitic infections. Thus, being immune suppressed can open the door to infection by an "opportunistic" agent like Mycobacterium tuberculosis or Staphylococcus. And it is this secondary infection that is often fatal.
In their recent Immunity paper, Oldstone and his colleagues report that the profound immunosuppression characterizing measles and lymphocytic choriomeningitis infections may occur because of a previously unrecognized interferon-induced signaling pathway in dendritic cells.
Dendritic cells have the potential to produce interferon in response to a viral presence, and the interferon binds to receptors on the same dendritic cells and on nearby cells. These receptors activate two signaling molecules called STAT1 and STAT2 (acronyms for “signal transducers and activators of transcription”). The two STATs then become linked together as heterodimer, and the interferon-activated STAT1/STAT2 heterodimers then translocate to the nucleus and turn on a number of other genes that cause the dendritic cells to grow, divide, and continue mediating an immune response.
However, interferon produced within dendritic cells during measles and lymphocytic choriomeningitis infections seems to induce STAT2 activation alone. Oldstone and his colleagues found that this STAT2-dependent, STAT1-independent signaling interferes with dendritic cell development and expansion in vitro and in vivo, giving the virus an advantage by reducing the immune response.
Because this mechanism was shared by two viruses from two different families, says Oldstone, this subversion of interferon’s action is something that may be common to a number of viruses that infect humans. Furthermore, the research suggests a possible new pathway to target for reducing the immunosuppressive effects of these viruses.
“Since you know what the pathway is that causes this suppression, you could [potentially] make drugs to block that pathway,” says Oldstone.
To read the article, “Viruses Evade the Immune System through Type I Interferon-Mediated STAT2-Dependent, but STAT1-Independent, Signaling” by Bumsuk Hahm, Matthew J. Trifilo, Elina I. Zuniga, and Michael B.A. Oldstone, see the February, 2005 issue of the journal Immunity or go to: http://dx.doi.org/10.1016/j.immuni.2005.01.00
Send comments to: jasonb@scripps.edu

In this month’s issue of the journal Immunity, Professor Michael B. A. Oldstone and Research Associates Matt Trifilo, Bumsuk Hahm, and Elina Zuniga (left to right) report that certain viruses have the ability to subvert a class of antiviral molecules called type I interferons, an important part of our innate resistance to viruses. Photo by Kevin Fung.
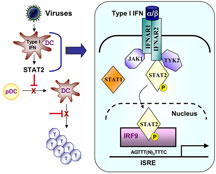
Viral infection of dendritic cells results in immune suppression. Click to enlarge
