

A Third Man Identified
By Jason Socrates Bardi
Mystery was never more aptly described as when it was called the "third man."
The phrase comes from a famous Graham Greene novel that was made into an even more famous 1949 film starring Orson Welles. The title The Third Man refers to an unknown character whose identity remains unknown through most of the narrative even though the other characters in the story deduce early on that he exists.
Modern science is filled with examples of what, for want of a better metaphor, can be termed "third men." These are things we know must exist even though we have not yet identified them. In astronomy, dark matter is a third man—we cannot see it, but we know it must encompass a sizable mass of the universe. In chemistry, the element gallium was a third man. When Dmitri Mendeleev constructed his periodic table in 1869, he predicted the existence of gallium based on the fact that there was a missing element in the periodic table.
In biology there are a lot of third men. The human genome is filled with "orphan" receptors that bind to as-yet-unidentified ligands. Similarly, as scientists elucidate key metabolic and signaling pathways in the body, they often come to realize that certain enzymes, substrates, and cofactors are involved before they can identity them.
Recently, Professor Gary Bokoch and his colleagues in the Department of Immunology at The Scripps Research Institute identified one of these third men—an enzyme called chronophin that is involved in regulating the dynamics of the actin cytoskeleton in cells. Actin dynamics plays important roles in fundamental cellular processes that include cell division, growth, and motility. These processes contribute to such diverse physiological situations as wound healing, angiogenesis, innate immunity, metastasis in cancer, and neuronal development.
A Regulator of Actin Dynamics
Scientists have for years sought the master regulators of processes like cell motility—the molecules that have their hands on the steering wheels and feet on the gas pedals. A few years ago, Bokoch and his colleagues found one of the master regulators of actin dynamics and motility—a small GTP binding protein called Rac that is found in the ruffles and edges of cells. The Bokoch group showed that Rac is spatially and temporally regulated to coordinate leading-edge extension and tail contraction during the "chemotactic" motility of human neutrophils, and showed that Rac is dynamically activated during motility.
Since then, the scientists have been trying to understand how Rac itself regulates the actin cytoskeleton. What are, for instance, the molecular switches that enable Rac to control the dynamic processes of cell motility?
About four years ago, they showed that one pathway that is regulated involves a protein called cofilin—an important cytoskeletal regulatory protein that controls the turnover of actin.
Cofilin acts both to sever actin filaments, generating new sites for actin polymerization in areas of rapid cell protrusion, and to promote actin depolymerization in areas of active turnover. Rac regulates cofilin's activity by controlling the attachment of a phosphate group to one of cofilin's critical serine residues. This does not occur through a direct effect of Rac, but involves the activation by Rac of a cascade of kinases termed p21-activated kinase 1 (Pak1) and LIM kinase. When cofilin is phosphorylated on the regulatory serine 3 site, it can no longer bind to actin filaments and is inactive.
The importance of cofilin phosphorylation in its regulation suggested the existence of another regulatory enzyme. If a phosphate group is attached through the action of Rac-stimulated kinases, a complementary "phosphatase" enzyme must remove this regulatory phosphate group and reactivate cofilin function. The existence of such a regulatory phosphatase was evident from studies that showed that the turnover of the phosphate group on cofilin was a dynamic and coordinated process.
The Third Man
One candidate early on was a protein called slingshot, which seemed to have the correct phosphatase activity. However, slingshot turned out not to be found in all organisms, nor ubiquitously distributed throughout the body, a requirement for the mystery phosphatase, as it would be needed wherever actin dynamics is taking place. Additionally, slingshot did not appear to be capable of regulating all cofilin-dependent cell processes, so clearly a third man still existed.
Now in an article recently published in Nature Cell Biology, Bokoch and his colleagues Antje Gohla and Jörg Birkenfeld argue that the third man phosphatase is actually chronophin. Taking the increasingly overlooked approach of function-based biochemical isolation, which required Gohla to spend significant time in the cold room, the scientists purified a phosphatase activity which dephosphorylated cofilin. This activity was then identified by mass spectrometry as chronophin, a member of a unique superfamily of proteins called haloacid dehydrogenases (HAD) normally associated with bacterial metabolism. Recently,however, an HAD phosphatase named "Eyes Absent" after the predominant phenotype observed when it is deleted in flies was shown to regulate transcription in mammalian cells. HAD family phosphatases use a unique chemical mechanism to abstract the target phosphate group from their substrates, and the Scripps Research scientists showed that chronophin shares this unusual mechanism.
Chronophin is ubiquitously distributed, can regulate actin turnover, and is present where actin is being rearranged. Chronophin activity affects cofilin phosphorylation levels and cofilin-dependent cytosletal processes. Importantly, chronophin contains the correct regulatory elements that allow it to interact in the circuit with Rac and cofilin to regulate cell motility. Moreover, Bokoch and colleagues also observed that the loss of chronophin activity in cells causes massive defects in cell division and leads to the creation of multinucleated cells.
While normally thought of in the context of cell motility, actin dynamics and cofilin activity are also necessary for coordinating the complex processes involved in the segregation of chromosomes during mitosis and cell division. The abnormal division of cells seen when chronophin function was altered is significant, as cell division defects are a hallmark of cancer. Many cancerous cells show signs of aneuploidy, where they have an unusual number of chromosomes and sometimes multiple nuclei. There is currently much debate as to the causes of aneuploidy and whether it is actually an initiating event in tumor formation.
The data from Bokoch and associates suggests the possibility that chronophin may be involved in the pathogenesis of malignant cancers such as neuroblastomas and germ cell seminomas, which are the most common form of solid tumors in children. Indeed, chronophin had been previously identified as an unknown protein upregulated in these aneuploid cancers.
More recently, anti-chronophin antibodies were identified in cancer patients. Such autoreactive antibodies are thought to contribute to the development of pathological conditions associated with the cancers. A better understanding of the role of chronophin in aneuploid cancers holds promise for the development of cancer drugs targeting this unique type of phosphatase.
The article, "Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics" by Antje Gohla, Jörg Birkenfeld, and Gary M. Bokoch appears in the January, 2005 issue of the journal Nature Cell Biology. See: http://dx.doi.org/10.1038/ncb1201
Send comments to: mikaono[at]scripps.edu

Professor Gary Bokoch and colleagues identified an enzyme that is involved in regulating the dynamics of the actin cytoskeleton in cells. Photos by Kevin Fung.
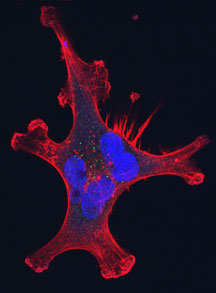
Disrupted actin cytoskeleton (red) and multi-nucleation (blue) resulting from siRNA-mediated knockdown of chronophin expression (green) in Hela cells.
