

Propagating Immunology
By Jason Socrates Bardi
A few years ago, shortly before Michael McHeyzer-Williams left Duke University to take a position as an associate professor at The Scripps Research Institute, he hired Laurent Malherbe as a research associate.
At the time, Malherbe was a recent graduate from the Ph.D. program in immunology at the University of Nice-Sophia Antipolis in France. He was interested in the questions related to adaptive immune and immunological memory that McHeyzer-Williams had been studying, and this common interest led Malherbe to McHeyzer-Williams's North Carolina laboratory.
When McHeyzer-Williams joined Scripps Research's Department of Immunology in 2001, Malherbe was one of three researchers who came with him to establish his new laboratory in the Institute for Childhood and Neglected Diseases building on the east side of campus.
From there the rest has been history. The two have worked closely together for the last few years, and, under McHeyzer-Williams's guidance, Malherbe has tackled some of the most difficult questions in the lab.
Through the last few years, says McHeyzer-Williams, Malherbe has developed into an outstanding young scientist, and their relationship has slowly grown from a simple mentor-trainee to one more closely resembles colleagues.
The latest fruit of their collaborative efforts appears in a recent issue of the journal Immunity, where McHeyzer-Williams and Malherbe describe a new way of looking at an area of immune research called clonal selection.
Clonal Selection in the Immune System
Over millions of years of evolution, the immune system has built up myriad ways of countering the countless potentially lethal bacterial and viral infections to which we, as organisms in the world, are exposed. One of the basic ways that the immune systems of creatures such as humans accomplish this is by producing cells that have the ability to recognize foreign antigens—pieces of protein or carbohydrate from a virus or bacterium that signify an invader.
Hundreds of millions of years ago, animals evolved innate ways of defeating pathogens, starting from the moment when they first enter the body and begin to circulate, infect, and replicate.
For instance, spider-like dendritic cells circulate through the bloodstream or sit in the skin or other organs where they are alert to the presence of pathogenic antigen. Once they encounter pathogenic antigen, they become active and mediate the general innate immune response and the clearance of the pathogens from the bloodstream and infected tissues. This involves making and releasing inflammatory chemicals and proteins that attract neutrophils and other "effector" innate immune cells that will kill the pathogenic invaders.
But we live in a world chock full of pathogens. And throughout evolution, the immune systems of higher organisms like mammals have faced a daunting problem: how could they possibly recognize all the myriad viral and bacterial antigens that are out there? Harder still, how could creatures' immune systems anticipate new antigens from mutated viruses and bacteria that don't even exist yet?
The answer that evolution came up with, at least in the case of humans and other mammals, was to develop an adaptive immune system with an extraordinarily large and diverse "repertoire" of adaptive immune cells. This repertoire is made up of millions of T and B cells that each have a unique receptor that enables them to recognize foreign antigens with extraordinary specificity.
These T and B cells circulate through the bloodstream and lymph nodes as naïve cells, and when the body is invaded by a foreign pathogen, those naïve T and B cells that are able to recognize the protein and lipid antigens unique to that foreign pathogen are multiplied, activated, and put to work killing the pathogen.
A single adaptive immune cell can proliferate into a million clones in a matter of days once it has been activated. These clones will all have the same unique receptor and the same ability to recognize the foreign antigen that stimulated the adaptive immune response to eliminate the antigen from the body.
But how does the body decide which B and T cells to propagate as clones? For B cells, the answer is straightforward.
B cells, which are responsible for producing antibody proteins during an adaptive immune response, follow a simple Darwinian path towards expansion and converge towards higher affinity. That is, the B cells that produce the antibodies that most tightly bind to the bacterial or viral antigen are the ones that are selected for expansion.
The B cells are selected for higher and higher affinity and those with the highest affinity are expanded the most.
This makes a lot of sense for B cells, says McHeyzer-Williams, because of what they do. "The primary purpose of B cells is to sniff out that really small amount of antigen," he says, and the tighter an antibody produced by a B cell binds to an antigen, the better that B cell will be at helping to clear the body of infection.
For years, McHeyzer-Williams adds, scientists assumed that T cell selection would follow more or less the same paradigm as B cells, but this turns out not to be the case. In fact, in their recent Immunity paper, McHeyzer-Williams and Malherbe describe how they found contrary behavior in one type of T cell known as the helper T cell.
Clonal Selection in T cells
Helper T cells are the master regulator of the adaptive immune system, initiating and regulating the entire adaptive immune response. They develop in the thymus and circulate as naïve cells until they encounter antigen on the surface of a professional antigen-presenting cell—a dendritic cell that takes up bacteria or viruses and chops their proteins and other antigen components into pieces and presents them on their surface in a large assembly known as the major histocompatability complex.
Each naïve helper T cell has its own unique T cell receptor that recognizes the antigen in the context of the major histocompatability complex, and once these T cells are activated, they will produce a swill of chemicals to clear the infection and to attract and activate other adaptive immune cells, like killer T cells and B cells.
To test how helper T cells are clonally selected, McHeyzer-Williams and Malherbe exposed rodent immune systems to an antigen composed of proteins taken from pigeons, which generally generate a robust immune response over the course of a week.
They used a technique called flow cytometry to tease apart the T cell population in the rodents at discrete time intervals after they were exposed to the antigen so that they could determine which T cells were undergoing expansion. As one would expect, T cells that do not have a minimal affinity for the antigen are not expanded.
This makes perfect sense—after all, why would the body waste time and energy propagating cells that are unable to participate in the immune response?
Biochemically this makes sense as well. A T cell undergoes expansion and activation as a result of a complicated series of gene expression and signaling events that all stem from the presence of an antigen bound to the cell's receptor. If the binding is not tight enough, the antigen will fall off too easily and the signaling events will not occur.
What was surprising to McHeyzer-Williams and Malherbe was that once T cells pass this threshold, the ones that bind the tightest do not seem to have an advantage over those that bind not so tightly.
They set up experiments to look for clonal competition after the first threshold, but every cell seemed to win.
Some get expanded until day three but then never make it beyond that. Some get expanded to day seven and become fully active effector cells. But this set varies in terms of their affinity for the ligand. Most surprisingly, some T cells that have the ability to bind foreign antigen tightly are not expanded as fully as other T cells that bind the same antigen less tightly.
In other words, unlike B cells, where expansion is simply proportional to binding affinity, any T cell that recognizes antigen with a certain minimal threshold affinity can be selected for expansion. Expansion seems to be proportional to the starting point from day three. The T cells expand beyond day three in proportion to how many there are at that time, and not according to how tightly they bind to antigen.
"This was really bizarre," says McHeyzer-Williams, "but now we can see that the basis of selection in the T cell compartment is an affinity threshold."
The situation is analogous to those entrance and exit exams that teenagers take to get out of high school and into college. The T cell threshold is like an exit requirement for graduation—one needs only score a minimal threshold to graduate and all students who demonstrate a simple competency in the subject matter will pass. B cell selection is more like the SAT—the higher the score, the better the chances of getting into college.
"You get over the threshold and everybody gets expanded," says McHeyzer-Williams.
The reason for this might be because T cells see foreign antigen in the context of the major histocompatability complex, which is a "self" molecule. If you keep selecting for higher affinity T cells, you are also selecting towards "self" reactivity, and potentially introducing autoimmune problems.
Full Circle
These results are pleasing for McHeyzer-Williams because they were produced after tremendous efforts on Malherbe's part to overcome some of the technological barriers involved.
"What Laurent has done is really push the system," says McHeyzer-Williams. "He does not shy away from technically difficult work, and it's satisfying for me to see this sort of progression in someone—in a young scientist."
And the results also validate the sort of cellular immunology that McHeyzer-Williams practices. "Some people think that what we do is a little old-fashioned—and it is conceptually," McHeyzer-Williams says. However, he adds, "Our methodology is extremely up to date"
To read the article, "Clonal Selection of Helper T Cells Is Determined by an Affinity Threshold with No Further Skewing of TCR Binding Properties" by Laurent Malherbe, Christina Hausl, Luc Teyton, and Michael G. McHeyzer-Williams, see the November 2004 issue of the journal Immunity or go to: http://dx.doi.org/
Send comments to: jasonb@scripps.edu

In a recent issue of the journal "Immunity", Christina Hausl, Laurent Malherbe, Michael McHeyzer-Williams, and Luc Teyton (not pictured), describe a new way of looking at the clonal selection of helper T cells.
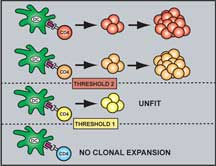
Schematic Representation of the TCR Affinity Thresholds Involved in Antigen-Driven Selection. Click to enlarge.
