Screen Yields Small Molecules that Target SARS Virus
By Jason Socrates Bardi
In the theatre of world health, severe acute respiratory syndrome (SARS) is a four-letter word—and one that entered our lexicon in dramatic fashion.
The disease, which is caused by a novel coronavirus that emerged in a remote region of southern China about 22 months ago, erupted in an outbreak in February 2003. The virus quickly spread to more than two dozen countries, infected some 8,000 people, and claimed nearly 800 lives before it was contained by that summer. The fact that the spread of SARS infections was contained is a testament to the good, old-fashioned public health strategies of monitoring, reporting, and isolating cases.
Today, the legacy of this successful effort to contain SARS can be found in the numerous pages devoted to aspects of SARS infections on the web sites of the World Health Organization and the U.S. Centers for Disease Control and Prevention (CDC). A recent review of these sites revealed detailed guidelines that cover everything from detecting and reporting new cases of SARS to guidance on the proper transport of SARS patients in airplanes.
Yet there was surprisingly little about how to treat SARS in the patients who have it. There are no specific drugs for SARS or for containing the SARS virus.
The CDC recommends that doctors give SARS patients "the same treatment that would be used for a patient with any serious community-acquired atypical pneumonia." For instance, a doctor might treat a SARS infection by administering ribavirin, an antiviral used to treat hepatitis C and infant pneumonia, and perhaps by administering corticosteroids to the patient as well. But there are no existing drugs available for treating SARS specifically—the way that there are drugs for treating HIV.
Recently, an effort to find other compounds that might be effective against the SARS virus was undertaken by a team of scientists at The Scripps Research Institute in La Jolla, California, and at Academia Sinica in Taiwan.
The effort, which was reported in a recent issue of the journal Proceedings of the National Academy of Sciences, was led by Scripps Research Professor Chi-Huey Wong, who holds the Ernest W. Hahn Professor and Chair in Chemistry and is a member of The Skaggs Institute for Chemical Biology at Scripps Research. The work is highlighted in the September issue of Nature Reviews: Microbiology.
Wong and his colleagues used a high-throughput screen in which they infected monkey kidney cells in culture with SARS and screened some 10,000 compounds for their ability to protect the cells from dying.
Included in these 10,000 compounds were a few hundred drugs that have already been approved by the U.S. Food and Drug Administration for treating other diseases, ginseng and about 1,000 other traditional Chinese herbs, several hundred chemicals that inhibit a class of enzymes known as proteases (the SARS virus has its own protease), and about 8,000 synthetic compounds, including aminoglycoside and oligosaccharide compounds Wong assembled using a technique he invented called programmable one-pot synthesis—a technique Wong uses to quickly assemble many types of carbohydrate structures.
Out of this library of 10,000 compounds, the scientists found about 50 that at reasonable concentrations offered the cells some protective effect against the SARS virus.
Several of these 50 were compounds that are either FDA-approved drugs in use to treat other conditions or are commonly used herbal supplements. And a few more are in the process of clinical development.
The protection exists in many of these cases because the compounds interfere with some part of the virus's lifecycle—such as the entry of the virus into a new cell or the assembly of new virus particles within an infected cell.
For instance, the SARS virus requires its own protease enzyme in order to assemble new virus particles, and Wong took that into account when he designed the 10,000-compound library, adding several protease inhibitors to the mix.
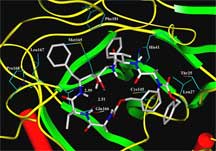
One of the compounds that most effectively inhibited the SARS virus was a protease inhibitor called TL3, which Wong described a few years ago with his Scripps Research colleagues John Elder, Art Olson, Bruce Torbett, and others. TL3 is an interesting molecule because it has the ability to effectively inhibit the proteases made by both human and cat immunodeficiency viruses. It surprised us, says Wong, that TL3 can also inhibit the SARS protease with Ki in the nanomolar range, even though the protease from SARS is quite different from HIV and FIV.
The article, "Small molecules targeting severe acute respiratory syndrome human coronavirus" by Chung-Yi Wu, Jia-Tsrong Jan, Shiou-Hwa Ma, Chih-Jung Kuo, Hsueh-Fen Juan, Yih-Shyun E. Cheng, Hsien-Hua Hsu, Hsuan-Cheng Huang, Douglass Wu, Ashraf Brik, Fu-Sen Liang, Rai-Shung Liu, Jim-Min Fang, Shui-Tein Chen, Po-Huang Liang, and Chi-Huey Wong was published by theProceedings of the National Academy of Sciences on July 6, 2004. Subscribers to the journal can access the article online at: http://dx.doi.org/10.1073/pnas.0403596101
For the research highlight of this article, see the September 2004 issue of Nature Reviews: Microbiology. Subscribers to the journal can access the article online at: http://www.nature.com/cgi-taf/DynaPage.taf?file=/nrmicro/journal/v2/n9/full/nrmicro985_fs.html
For more general information on SARS see: http://www.cdc.gov/ncidod/sars/ and http://www.who.int/csr/sars/en/
Send comments to: jasonb@scripps.edu
Computer modeling of TL-3 binding to SARS-CoV 3CL protease. Click to Enlarge.