DESIGN OF AN INTERLOCKED PROTEIN
DESIGN OF AN INTERLOCKED PROTEIN |
|
 |
|
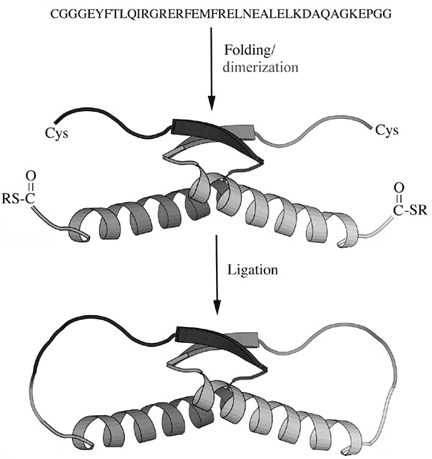
| A linear peptide corresponding to the p53 tetramerization domain was synthesized with an N-terminal cysteine residue and a C-terminal thioester group. Following folding of the polypeptide into a intersecting conformation (shown as a dimer for clarity), the ends of each peptide were closed by a specific chemical ligation reaction. The interlocking catenane product was conformationally similar to the linear folded protein but was extremely stable against thermal denaturation. |