Signaling by G Protein Coupled Receptors

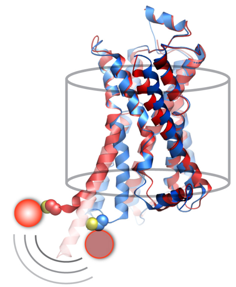
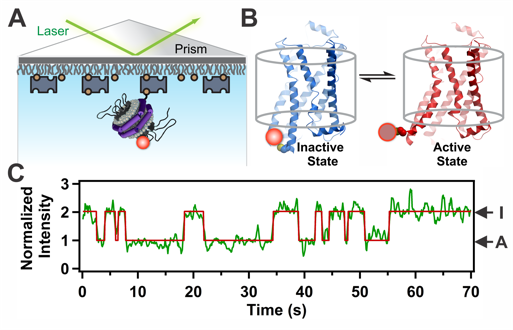
G Protein-Coupled Receptors (GPCRs) are remarkable allosteric machines that bind many types of ligands on their extracellular face and undergo conformational changes that initiate signal transduction pathways inside the cell. GPCRs are expressed in almost all human tissues and play a key role in human physiology and disease. Accordingly, GPCRs are the targets for more than 30% of pharmaceutical drugs on the market today. We have devised a new single-molecule spectroscopic method to characterize the conformational response of a GPCR upon binding pharmacologically distinct ligands. We use the human beta 2-adrenergic receptor (β2AR) as a model GPCR. To visualize β2AR at the single-molecule level, we label the protein with a fluorophore at the cytoplasmic end of transmembrane helix 6 and reconstitute single receptor molecules into phospholipid nanodiscs, which are then immobilized on a microscope slide and illuminated with a laser (Fig. 1). The fluorescence signal fluctuates over time between two distinct intensity states, corresponding to inactive and active receptor conformations. These fluctuations are observed even in the absence of drug ligands, explaining why β2AR exhibits a significant degree of ligand-independent (basal) signaling activity. Notably, binding of agonist ligands accelerate switching to the active conformation and retard the return to the inactive conformation, whereas inverse agonists act in the opposite manner. These studies open a new window on the dynamic behavior of this important class of signaling proteins and reveal that drug ligands operate by fine-tuning the conformational dynamics of the receptor. This knowledge should help in the design of improved drugs with predictable and finely tuned therapeutic effects.
Figure 1. Conformational transitions of β2AR at the single-molecule level. A. An individual labeled receptor molecule in a nanodisc immobilized on a quartz surface. B. Expanded view of a single receptor in inactive (I) and active (A) conformations. C. Normalized fluorescence intensity trajectory (green) and fitted lines (red), showing fluctuations between inactive and active states.
Recent Millar Laboratory Publications
R. L. Lamichhane, J. Liu, S. Katritch, K. L. White, R. C. Stevens, K. Wüthrich and D. P. Millar, “Biased Signaling of the G Protein-Coupled Receptor β2AR is Governed by Conformational Exchange Kinetics”, Structure 28, 371-377 (2020). PMID: 31978323.
R. L. Lamichhane, J. Liu, R. Pauszek III and D. P. Millar, “Fluorophore Labeling, Nanodisc Reconstitution and Single-Molecule Observation of a G Protein-Coupled Receptor”, Bio-Protocol, 7, e2332 (2017). PMID: 29170748.
R. Lamichhane, J. J. Liu, G. Pljevaljcic, K. L. White, E. van der Schans, V. Katritch, R. C. Stevens, K. Wüthrich and D. P. Millar, "Single-Molecule View of Basal Activity and Activation Mechanisms of the G Protein-Coupled Receptor β2AR", Proc. Natl. Acad. Sci. USA 112, 14254-14259 (2015).
Thursday, March 24, 2016