

Molecular Component of Innate Immunity Discovered
By Jason Socrates Bardi
“There is no need to be a doctor or a scientist to wonder why the human body is capable of resisting so many harmful agents in the course of everyday life,” the great Russian immunologist Ilya Mechnikov stated in his 1908 Nobel prize acceptance speech. And indeed he was right—how in the world do we survive?
Humans, like all higher organisms, are constantly challenged in a world filled with microbial pathogens. We are bathed in bacteria, confronted with fungi, pilloried with parasites, and invaded by viruses. And yet, most of the time, we survive.
One reason we do, as Mechnikov was one of the first to discover, is that we possess an ancient and crucial physiology known as innate immunity that is active in eukaryotic organisms as diverse as humans and fruit flies. In fact, Mechnikov discovered innate immunity in starfish, when he observed “phagocytic” starfish cells such as macrophages, which could engulf and destroy foreign pathogens.
In the century that followed Mechnikov’s work, much of the broad picture of innate immunity has become clear. Broadly speaking, the recognition of foreign antigen triggers the immune system, which responds with a multi-stage biochemical defense starting with the unleashing of an army of white blood cells, like macrophages, which engulf and destroy pathogens. The macrophages also fight the pathogens by producing large amounts of chemicals that induce inflammation and help the body clear the infection.
But many of the details of the molecules and signaling pathways that allow this vigorous immune defense are still being elucidated.
Now, in a paper appearing in this week’s issue of the journal Nature, Professor Bruce Beutler, Research Associate Kasper Hoebe, and their colleagues at The Scripps Research Institute in La Jolla, CA have identified one of the molecules that mediates innate immune recognition—CD36.
Uncovering CD36’s Role
Normally, when human or mouse cells encounter bacteria or viruses, they recognize proteins, lipids, or other molecular components of these foreign invaders through a family of receptor proteins called the toll-like receptors (TLRs). Humans have at least 10 different TLRs, and one of Beutler’s goals is to identify how these receptors and associated immune molecules such as CD36 mediate innate immunity.
One way he has approached this is through a technique known as positional cloning, which combines classical genetic mapping methods with high-powered computer-aided searches to identify relevant genes.
"We're attempting to create mutations that destroy innate immunity and in this way, to identify all of the genes involved in innate immunity or at least as many as we can,” Beutler says. “[When] we find a model that is immunocompromised we can go back and positionally clone the critical gene that we have hit."
About two years ago, they detected a genetic mutation termed oblivious that renders its carriers’ macrophages unable to detect a molecule called MALP-2, which is produced by the bacterium Mycoplasmapneumoniae, and unable to detect a crude preparation of commercial peptidoglycan derived from the bacterium Staphylococcus aureus.
This mutation weakened the innate immune systems of the mice, which are immunodeficient and highly susceptible to bacterial infections. Using positional cloning, Beutler, Hoebe, and their colleagues mapped the oblivious mutation to an obscure gene CD36, which has an equivalent gene in humans
CD36 is produced by various types of cells associated with the blood and the immune system. It can be found expressed in mammals both on the surface of blood platelets and on endothelial cells lining the blood vessels, where it is used for platelet adhesion. Previously, CD36 had been identified on the surface of innate immune cells as a class II scavenger—it scavenges or removes potentially dangerous endogenous molecules from the body, such as human proteins that have become oxidized or cross-linked.
Given CD36’s role in recognizing both endogenous human molecules and exogenous bacterial molecules, Beutler, Hoebe, and their colleagues suggest that it may be a mediator of what is known as a sterile inflammation, in which immune cells release inflammatory chemicals in the absence of any infection. Sterile inflammation is a condition common to many different diseases, including autoimmune diseases.
It is still not clear whether TLRs are involved in sterile inflammation, but CD36’s involvement strongly suggests that they may be. If that is the case, then scientists will have a potentially valuable target for the design of drugs aimed at treating diseases involving sterile inflammation. By designing a way to block some part of the TLR signaling pathway, they might succeed at reducing sterile inflammation and ameliorating some of the diseases it causes.
The article, “CD36 is a sensor of diacylglycerides” is authored by Kasper Hoebe, Philippe Georgel, Sophie Rutschmann, Xin Du, Suzanne Mudd, Karine Crozat, Sosathya Sovath, Louis Shamel, Thomas Hartung, Ulrich Zähringer, and Bruce Beutler and appears in the February 3, 2005 issue of the journal Nature. See: http://www.nature.com/cgi-taf/DynaPage.taf?file=/nature/journal/v433/n7025/full/nature03253_fs.html. This work was supported by the National Institutes of Health.
Send comments to: jasonb@scripps.edu

"We're attempting to create mutations that destroy innate immunity and in this way, to identify all of the genes involved…” says Professor Bruce Beutler.
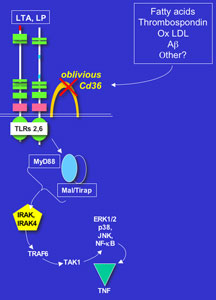
Signaling pathway in the innate immune system revealed. Click to Enlarge.
