

Sinister Amino Acids or Innocent Victims of Evolution?
By Jason Socrates Bardi
Growing up left-handed may not be as difficult today as it was in past eras, when it was regarded as a mark of nefariousness (the word "sinister," in Latin, means left).
Nevertheless, as schools across the country commence their first classes this month, a certain percentage of the children will be discovering that there remain difficulties to growing up left-handed in a predominantly right-handed world—too few lefty desks in most classrooms, for instance.
Fortunately proteins never have such problems.
Proteins, like other biological polymers, are composed of building blocks that have "chiral" centers—something that has come to be called handedness—in which the atoms are arranged asymmetrically such that the molecule cannot be superimposed on its mirror image (just as a left hand cannot be superimposed on top of a right hand). All the amino acid building blocks from which natural proteins are made are, in fact, left-handed.
But why? Right-handed amino acids are no less stable and no more reactive, so why does nature use only left-handed amino acids to make proteins?
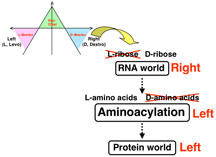
One possible answer to this question may lie several billion years back in what scientists theorize may have been an early, pre-protein RNA world. According to this theory, there was an ancient RNA world in which RNA enzymes were the chief catalytic molecules and RNA nucleotides were the building blocks that stored genetic information.
Later, the theory goes, as the RNA world was evolving into the modern DNA–RNA–protein world, RNA molecules began to string together amino acids into proteins. Proteins are much more versatile chemically and would have been advantageous to RNA-based life forms that could synthesize them. And modern protein synthesis, the stringing together of amino acids, is the legacy of this early RNA world.
While this has been an enticing argument for a number of years, nobody had ever produced experimental support of the concept. But now Scripps Research Associate Koji Tamura and Professor Paul Schimmel, who is the Ernest and Jean Hahn Professor and Chair of Molecular Biology and Chemistry and is a member of The Skaggs Institute for Chemical Biology at The Scripps Research Institute, have done just that.
In an article is a recent issue of the journal Science, Tamura and Schimmel describe how they synthesized an RNA-based system for aminoacylation (attaching an amino acid to an RNA molecule) using both left- and right-handed RNA.
The sugar backbone of RNA chains are chiral—they are only made of right-handed ribose sugars—and the chirality of the evolutionarily older RNA may have determined the chirality of the amino acids used to make the evolutionarily younger proteins. The RNA molecules that were grabbing amino acids were right-handed, and in the system reconstructed by Tamura and Schimmel, this caused them to preferentially select amino acids that were left-handed.
They found experimentally that right-handed RNA preferred left-handed amino acids and the mirror image left-handed RNA preferred right-handed amino acids. This supports the notion that nature's preference for left-handed amino acids was determined by the handedness of RNA in that early, pre-protein RNA world.
Still, knowing that lefties dominate the micoscopic scene may be of little comfort to today's lefty school children who find themselves relegated to those lonely, weathered, left-handed desks in the corner.
The article "Chiral-Selective Aminoacylation of an RNA Minihelix" by Koji Tamura and Paul Schimmel appears in the August 27, 2004 issue of the journal Science. See: http://dx.doi.org/10.1126/science.1099141.
Send comments to: jasonb@scripps.edu
Selective incorporation of L-amino acids. Click to enlarge.
