Versatile Optically Active Products from a Simple Catalyst
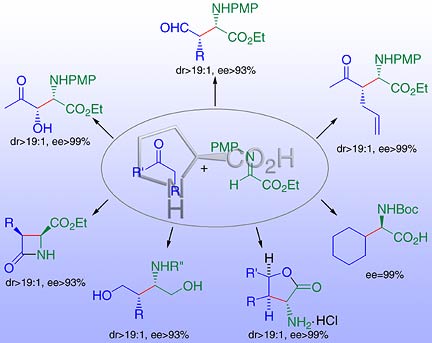
The figure shows the amino acid proline acting as catalyst for the reaction of an enolizable carbonyl donor (aldehyde or ketone, blue) with an acceptor imine (green). Depending on the type of donor used, an assortment of enantiomerically pure products can be made, including syn-b-formyl-a-alkyl substituted amino acids, a-amino-d-lactones, b-lactams, and 2-amino-1,4-diols.