Of Drugs and Diseases:
GCRC Provides Site for Clinical Studies
By Mika Ono
Hairy cell leukemia, a rare form of chronic leukemia, can be fatal.
But thanks to research conducted by investigators of The Scripps Research
Institute (TSRI) in its General Clinical Research Center (GCRC), a seven-day
course of 2CdA, an intravenous medication with remarkably few side effects,
now cures or produces many years of freedom from disease in almost all
those receiving treatment.
The GCRC’s role in bringing 2CdA—marketed under the name cladribine
(Leustatin) by Ortho Biotech, Inc., an affiliate of Johnson&Johnson—from
bench to bedside throughout the ‘80s and ‘90s is perhaps the
most dramatic example of the GCRC’s success. But it is by no means
the only one.
“The GCRC enables researchers to test the clinical utility of discoveries
made in the lab,” says Program Director Francis Chisari, who has
himself conducted research at the facility with the goal of developing
therapeutic vaccines to terminate chronic hepatitis B and C virus infections.
"The GCRC provides a forum to study the underlying mechanisms of illnesses—such
as thrombotic and cardiovascular diseases, alcoholism, infectious diseases,
sleep disorders, allergic and autoimmune diseases, neurogenerative diseases,
and cancer—as well as to test the safety and efficacy of potential
small-molecule drug treatments."
Located in a wing of the Scripps Green Hospital (although still part
of TSRI), the GCRC provides investigators with:
- a seven bed inpatient unit, including a sleep lab;
- an adjacent outpatient suite;
- nursing staff specially trained to provide both excellent patient
care and rigorous research data collection;
- a core laboratory staffed and equipped to perform specialized research
assays and provide specimen preparation and storage; and
- a computer center, staffed with a biostatistician and a systems manager,
to help investigators design studies and perform sophisticated genetic
and clinical data management and analysis.
In addition, the GCRC runs a forward-looking normal blood donor program,
which ensures that the blood used in laboratory experiments is properly
drawn, screened, and categorized. "We have a health care professional
who draws blood from a pool of 200 to 300 donors," says the GCRC’s
Administrative Manager Beth Bieger. "We are able to provide a medically
controlled environment for the procedure, protect the anonymity of donors,
and screen the blood for disease. This is a valuable service—a much
better alternative than having a post-doc roll up his or her sleeve every
time blood is needed in the lab."
The GCRC is funded by a competitively renewed grant from the National
Institutes of Health (NIH) and supplemented by founding donations from
the William Black family and continuing support from the Stein Endowment
Fund.
"The GCRC’s NIH grant will support the clinical aspects of an investigator’s
research—even if his or her research grant does not have a clinical
budget," Chisari notes. "The GCRC can pick up the tab for things like
documenting a disease state or monitoring clinical parameters that may
be relevant to the fundamental question under investigation. It also provides
a licensed hospital environment for research subjects to be monitored,
for tissue and body fluids to be collected, and for observational and
interventional studies to be performed in an outpatient setting."
Any researcher who wishes to conduct a study at the GCRC must submit
a protocol to the Human Subjects Committee (independent of the GCRC),
which reviews the safety, ethical, and human-protection aspects of the
study. If the protocol passes, the study is then reviewed by the GCRC
Scientific Advisory Committee, which evaluates the proposal for scientific
merit before granting access to the facility. There are currently some
80 active protocols at the GCRC, all of which have passed this rigorous
review.
In addition to supporting studies by TSRI investigators, the GCRC is
open to clinicians in The Scripps Hospital and Medical Group interested
in conducting research. When studies are sponsored by a for-profit entity,
such as a pharmaceutical company, the cost to the GCRC is reimbursed in
full.
Ernest Beutler, professor and chair of the Molecular and Experimental
Medicine Department, is a TSRI investigator who has made ample use of
the facility during his 21 years at the institute. Beutler initiated the
initial 2CdA studies on leukemia and lymphoma—and subsequent studies
that showed the compound effective in the treatment of multiple sclerosis.
Most recently, he conducted research there showing that the cost of effectively
treating Gaucher’s disease could be reduced from $500,000 to $80,000
per year by using a lower dose of the recommended drug.
"When I first came to TSRI the economic situation in health care was
not as desperate as it is today," Beutler comments. "It was still possible
to carry out studies in a hospital without paying a prohibitive amount
for a bed. Today, the GCRC is the only way TSRI researchers can carry
out independent clinical studies. The GCRC is absolutely key."
Go back to News & Views Index
|

Registered Nurses Janine Kampleman (left) and Kristen
Greiner process patients’ specimens in the Core Laboratory located
on the GCRC nursing unit in the Green Hospital.
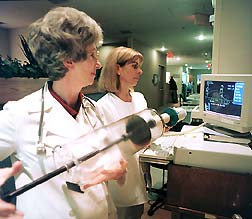
Registered Nurses Helen Darnley (left) and Susan
Dastrup perform the daily calibration of the pulmonary function equipment,
used to measure the lung capacity of patients participating in any of
several allergy studies.
|



